

Mechanobiology and Mechanotherapy Laboratory
Mechanobiology and Mechanotherapy Laboratory
News
Comming soon!
Laboratory Mission, Principles, and Agenda
1.We aim to make meaningful contributions to the field of medicine by developing novel treatment strategies, methods, and technologies.
2.The laboratory prioritizes the exchange of common interests, ideas, and aspirations between individuals and between education and research institutions, i.e., universities, medical institutions, and private companies.
3.Our laboratory is currently developing “Mechanotherapies” that control the physical microenvironment of cells. We focus on “Mechanobiology”, which is the study of how cells, tissues, and organs sense mechanical forces and regulate their vital activities in response to these forces.
4.Traditional physical therapy focuses primarily on rehabilitation; however, recent developments in mechanobiology have highlighted the effects of physical forces on cells, tissues, and organs. Thus, “old” therapy models need to be updated. Our laboratory is working on ways of repairing and regenerating injured/aged tissues and organs by controlling the mechanical forces to which they are subjected.
5.Moreover, we are studying the mechanisms underlying mechanobiology-related disorders/diseases in physical microenvironments that are “out-of-control”, i.e., keloids, with the aim of developing suitable treatment strategies.
Laboratory members
Laboratory Director
-

Rei Ogawa, M.D., Ph.D., F.A.C.S.
https://sites.google.com/view/rei-ogawa-en
Biography
1999 Resident, Department of Plastic, Reconstructive, and Aesthetic Surgery, Nippon Medical School, Tokyo, Japan 2002 Instructor, Department of Plastic, Reconstructive, and Aesthetic Surgery, Nippon Medical School, Tokyo, Japan 2005 Head, Department of Plastic Surgery, Aidu Chuo Hospital, Fukushima, Japan 2006 Assistant Professor, Department of Plastic, Reconstructive, and Aesthetic Surgery, Nippon Medical School, Tokyo, Japan 2007 Research Fellow, Wound Healing and Tissue Engineering laboratory, Division of Plastic Surgery, Department of Surgery, Brigham and Women’s Hospital, Harvard Medical School, Boston, USA 2009-2013 Associate Professor, Department of Plastic, Reconstructive, and Aesthetic Surgery, Nippon Medical School, Tokyo, Japan Principal Investigator, Mechanobiology and Mechanotherapy Laboratory, Nippon Medical School, Graduate School of Medicine, Tokyo, Japan 2013-Present Visiting Lecturer, Department of Plastic Surgery, Tokyo University 2015-Present Chief and Professor, Department of Plastic, Reconstructive and Aesthetic Surgery, Nippon Medical School -

Principal Investigators:PI
Hiroya Takada, Ph.D.
Biography
1997-1999 Research Fellow of Japan Society for the Promotion of Science (JSPS), Department of Energy and Hydrocarbon Chemistry, Graduate School of Engineering, Kyoto University, Japan 1999 PhD of Engineering, Department of Energy and Hydrocarbon Chemistry, Graduate School of Engineering, Kyoto University, Japan 1999-2000 Research Fellow, Department of Chemistry, University of Warwick, UK 2000-2002 Research Fellow, Centre National de la Recherche Scientifique, Ecole Normale Supérieure de Paris, France 2003-2006 Mitsubishi Corporation/VC60 BioResearch Corparation, Japan 2006-2014
2015-2017Pixy Corporation, Central Institute Deputy Director, Japan 2009 Research Fellow, Department of Physiology, Graduate School of Medicine Nagoya University, Japan 2014 PhD of Medicine, Department of Physiology, Graduate School of Medicine Nagoya University, Japan 2014-2015 L’Oréal Japan, Applied Research Manager 2017 Research Fellow of Japan Agency for Medical Research and Development (AMED), Department of Plastic, Reconstructive and Regenerative Surgery, Nippon Medical School Graduate School of Medicine, Japan 2017-2018 Lecturer, Department of Plastic, Reconstructive and Regenerative Surgery, Nippon Medical School Graduate School of Medicine, Japan 2019-Present Associate Professor, Anti-Aging & Preventive Medicine, Nippon Medical School Graduate School of Medicine, Japan -

Takunori Ogaeri, Ph.D.
Biography
2009 PhD of Medicine, Department of Stem Cell Biology and Regenerative Medicine, Tokyo University Graduate School of Medicine, Japan 2009-2011 Research fellow: Biomedical Research Institute, National Institute of Advanced Industrial Science and Technology (AIST) 2011-2016 Research fellow: Department of Organ Regeneration Medicine, Yokohama City University Graduate School of Medicine. 2016-2019 Research fellow: Mechanobiology Lab, Nagoya University Graduate School of Medicine. 2019-2021 Research fellow: Department of Molecular Pharmacokinetics, Graduate School of Pharmaceutical Sciences, University of Tokyo. 2021-2024 Assistant Professor: Department of Medical Research and Development for Oral Disease, Graduate School of Biomedical Sciences, Nagasaki University. 2024-Present Associate Professor: Anti-Aging & Preventive Medicine, Nippon Medical School Graduate School of Medicine, Japan -

Teruyuki Dohi, M.D., Ph.D.
Biography
2005 Junior Resident, Nippon Medical School, Tokyo, Japan 2007 Senior Resident, Department of Plastic, Reconstructive and Aesthetic Surgery, Nippon Medical School, Tokyo, Japan 2009 Aidu Chuo Hospital, Fukushima, Tokyo
Instructor, Department of Plastic, Reconstructive and Aesthetic Surgery, Nippon Medical School, Tokyo, Japan20015 Chief in Plastic, Reconstructive and Aesthetic Surgery, Towa Hospital, Tokyo, Japan 2016 Instructor, Department of Plastic, Reconstructive and Aesthetic Surgery, Nippon Medical School, Tokyo, Japan 2016-2018 Research Fellow, Division of Plastic Surgery, Stanford University, CA, USA 2018-2019 Clinical Assistant Professor, Department of Plastic, Reconstructive and Aesthetic Surgery, Nippon Medical School, Tokyo, Japan 2019-Present Assistant Professor in the Department of Plastic, Reconstructive and Aesthetic Surgery, Nippon Medical School, Tokyo, Japan
Adjunct Research Fellow
-

Satoshi Akaishi, M.D., Ph.D.
Biography
2000 Resident, Department of Critical Care Medicine, Nippon Medical School, Tokyo, Japan 2002 Senior Resident, Department of Plastic, Reconstructive and Aesthetic Surgery, Nippon Medical School, Tokyo, Japan 2005 Head, Department of Plastic Surgery, Aidu Chuo Hospital, Fukushima, Japan 2008 Instructor, Department of Plastic, Reconstructive, and Aesthetic Surgery, Nippon Medical School, Tokyo, Japan 2010 Research Fellow, Division of Plastic Surgery, Stanford University 2017 Assistant Professor, Department of Plastic, Reconstructive, and Aesthetic Surgery, Nippon Medical School, Tokyo, Japan 2020-Present Professor, Nippon Medical School Musashikosugi Hospital -

Chenyu Huang, M.D., Ph.D.
Biography
2002 Resident, Beijing Jishuitan Hospital, Beijing, China 2008 Attending Physician, Meitan General Hospital, Beijing, China 2009 Visiting Scholar, Nippon Medical School Hospital, Tokyo, Japan 2011 Associate Chief Physician, Meitan General Hospital, Beijing, China 2013 Postdoc, Brigham and Women's Hospital, Boston, US 2014 Associate Chief Physician, Beijing Tsinghua Changgung Hospital, Beijing, China 2019 Associate Professor, Tsinghua University, Beijing, China 2022-Present Chief Physician, Beijing Tsinghua Changgung Hospital, Beijing, China -

Mido Abdelhakim, M.D., Ph.D.
Biography
2015 Resident, School of Medicine, Cairo University, Cairo, Egypt 2016 Clinical Clerkship, Department of Dermatology & Plastic Surgery, St. Luke’s International Hospital, Tokyo, Japan 2017 Ph.D. Candidate, Department of Plastic, Reconstructive and Regenerative Medicine, Graduate School of Nippon Medical School, Tokyo, Japan 2021 Postdoctoral Scholar, Plastic, Reconstructive and Aesthetic Surgery, Nippon Medical School Hospital, Tokyo, Japa 2024-Present Visiting Instructor, Stanford Technology Training, Healthcare & Medical Education, Stanford, CA
Research
The three-dimensional structure of living things is influenced by various physical factors, including gravity, atmospheric pressure, and water pressure. It is believed that the environment, particularly the physical environment, had a great impact on the beginnings of life on the earth, and on the development of multi-celled organisms from single-celled organisms.
Within the human body, the musculoskeletal system (which includes bones, cartilage, tendons, and muscles) grows/develops to support our body weight. At the same time, the skin stretches to accommodate this growth. The heart beats constantly, blood flows through vessels, and the lungs expand and contract as we breathe. Every part of our body is stretching/shrinking/moving as we perform our daily activities. Thus, mechanical forces are necessary to maintain the structure and function of our cells, tissues, and organs.
At the cellular level, cells change shape, and mass transfer occurs between the inside and outside of the cell membrane; these processes are driven by mechanical forces. Such mechanical force-mediated biological processes regulate gene expression and play an important role in the life-cycle of the cell. “Mechanobiology”, the study of how cells sense mechanical forces, aims to identify how cells are influenced by the microenvironment. For example, a paper published in the Journal of Cellular and Molecular Medicine suggests that there is a possibility that various skin disorders, including keloids, can be treated by mechanobiological methods. Moreover, mechanobiology-mediated medicine can be used to analyze/treat many disorders/diseases, accelerate wound healing, achieve scarless wound healing, and repair and regenerate injured/aged tissues and organs. Also, our group is currently attempting to regenerate nail and hair, and is conducting research into the medical fields of aesthetics and anti-aging.
We defined mechanobiology-mediated medicine (the control of mechanical forces) as “Mechanotherapy” and published the concept in “Trends in Molecular Medicine”. Traditionally, mechanotherapy has meant “physical therapy” and the rehabilitation of muscles. However, we have re-defined “mechanotherapy” for a new medical era; we believe that it now encompasses the control of mechanical forces that act on cells, tissues, and organs. Indeed, the concept may even be expanded to encompass the effects of mechanical forces at the molecular level. At present, we study mechanobiology and mechanotherapy at both the basic research and clinical levels.
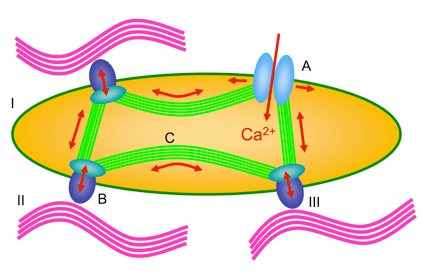
Mechanisms by which cells sense mechanical forces (mechanosensors)
I: cell, II: extracellular matrix, III: cell adhesion molecules
A: mechanosensitive ion channels, B: integrins, C: actin filaments
The life of a cell is regulated by mechanical forces that are “sensed” by cellular mechanoreceptors,i.e.,mechanosensitive ion channels, cell cytoskeletal components (actin), and cell adhesion molecules expressed by the cells.
Ogawa R. Keloid and hypertrophic scarring may result from a mechanoreceptor or mechanosensitive nociceptor disorder. Med Hypotheses. 2008 Oct;71(4):493-500.
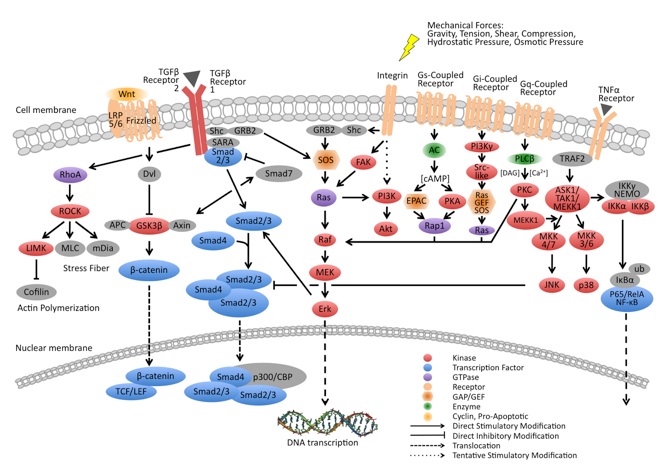
Mechanisms underlying altered gene expressions in cells after the sensing of mechanical forces
(mechanosignaling pathways)
Various signaling pathways are up- or down-regulated in response to mechanical forces perceived by mechanoreceptors. These signaling pathways are called mechanosignaling pathways, and are closely related to the mechanisms underlying disorders/diseases.
Huang C, Ogawa R. Fibroproliferative disorders and their mechanobiology. Connect Tissue Res. 2012;53(3):187-96.
Huang C, Holfeld J, Schaden W, Orgill D, Ogawa R. Mechanotherapy: revisiting physical therapy and recruiting mechanobiology for a new era in medicine. Trends Mol Med. 2013.
Developement of Mechanotherapy Techniques for Regenerating Skin and Accelerating Wound Healing
Tissue-level mechanotherapies mainly focus on improving wound healing and include negative pressure wound therapy (NPWT) = microdeformational wound therapy (MDWT), shockwave therapy, soft tissue expansion, distraction osteogenesis and surgical tension reduction.
The laboratory are developing devices of mechanotherapy with private companies for regenerating skin and accelerating wound healing.
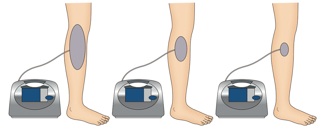
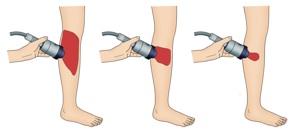
Negative Pressure Wound Therapy and Extracorporeal Shock Wave Therapy
MDWT, also called vacuum-assisted closure (V.A.C.) or negative pressure wound therapy (NPWT) and extracorporeal shock wave therapy are good examples of mechanotherapy for regenerating skin and accelerating wound healing.
NPWT involves the application of a vacuum to a wound surface by packing the wound with a porous polyurethane sponge and then sealing the wound with occlusive dressing connected to the vacuum. It is effective for treating acute wounds as well as chronic, open, large and contaminated wounds. Its effects are largely the result of removing extracellular fluid, stabilizing the wound environment, generating contracture of the wound or macrodeformation, and inducing microdeformation at the foam-wound interface.
The shockwaves that are commonly used in extracorporeal shockwave therapy (ESWT) are biphasic high-energy acoustic waves that can be generated by electrohydraulic, electromagnetic or piezoelectric technologies. ESWT is a well-known lithotripsy method whose potential in promoting wound healing is currently being investigated vigorously.
Qureshi AA, Ross KM, Ogawa R, Orgill DP. Shock wave therapy in wound healing. Plast Reconstr Surg. 2011 Dec;128(6):721e-7e.
Erba P, Ogawa R, Ackermann M, Adini A, Miele LF, Dastouri P, Helm D, Mentzer SJ, D'Amato RJ, Murphy GF, Konerding MA, Orgill DP. Angiogenesis in wounds treated by microdeformational wound therapy. Ann Surg. 2011 Feb;253(2):402-9.
Developement of Mechanotherapy Techniques for Regenerating Skin Appendages (Nails and Hairs)
Working in our laboratory, Dr. Hitomi Sano showed that nails maintain their structure via mechanical forces generated by a process known as “grasp and walk”. For example, it is thought that anemia or drugs can induce koilonychias. However, anemia or drugs only cause thinning or weakening of the nail matrix; nail deformity is produced by mechanical forces. Moreover, poorly fitting shoes, injury, nails that are too short, and inherited factors were all thought to cause curved or ingrowing toe nails. Paradoxically, however, we found that bedridden and non-ambulatory patients are at high risk of suffering from these conditions. Thus, it appears that normal toe nail configuration is maintained by counter pressure generated by the floor, and that the mechanical forces on the foot generated by standing and walking are necessary for normal toe nail development and function.
We are also studying the effects of mechanical forces on hair growth and regeneration with the help of Angfa Corporation (famous for their product, Scalp-D). We found that hair papillary cells sense mechanical forces, and that hair growth-related gene expression is up-regulated by stretching tension. We have established a business-academic collaboration to study the repair and regeneration of skin appendages using mechanotherapy.
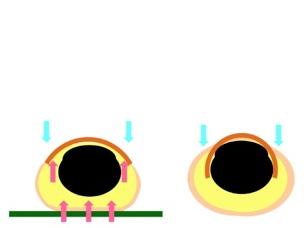
Mechanobiological methods to regenerate nails and hair
Mechanical forces generated by standing and walking are necessary to maintain the normal configuration and function of toe nails. Therefore, bedridden and non-ambulatory patients are at high risk of suffering from curved or ingrown toe nails. Hair papillary cells can sense mechanical forces, and hair growth-related gene expression is up-regulated by stretching tension.
また、右図に示すように、毛乳頭細胞に伸展刺激を加えると、メカノシグナル伝達経路を介し、発毛促進に関連する遺伝子発現が増強することを明らかにしてきた。
Sano H, Ogawa R. Role of Mechanical Forces in Hand Nail Configuration Asymmetry in Hemiplegia: An Analysis of Four Hundred Thumb Nails. Dermatology. 2013 Jun 22.
Mechanical Loading to Regenerate Bone and Cartilage using Stem Cells
Body weight and walking subject the articular cartilage of the knee to high hydrostatic pressures, which are generated by the articular fluid. When the body weight is loaded onto the articular capsule it can generate a force of up to 30 atmospheres. Therefore, if our aim is to grow/regenerate articular cartilage under ex vivo conditions, it is clear that we cannot generate this level of pressure in a culture dish. Thus, in a study supervised and conducted at Brigham and Women’s Hospital, Harvard Medical School, Dr. Shuichi Mizuno used bioprocessors or bioreactors to simulate and generate the high hydrostatic pressures required. He succeeded in generating high quality cartilage from adipose-derived stem cells under these high hydrostatic pressure loading conditions.
We are the first group to show that not only cartilage regeneration, but also bone regeneration, is accelerated by high hydrostatic pressures. We found that subjecting mesenchymal stem cells in hydroxyapatite scaffolds to high hydrostatic pressures led to the regeneration of the bone matrix. We believe that this is very interesting work because it appears that bone does not normally “sense” hydrostatic pressure within our bodies. However, differentiated osteocytes do respond to hydrostatic pressure. It is thought all the cells in our body can potentially “sense” mechanical forces and respond to them. Further research into the mechanosensitivity of cells is required.
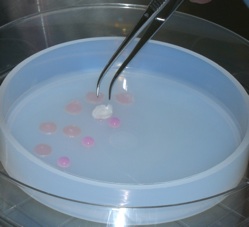
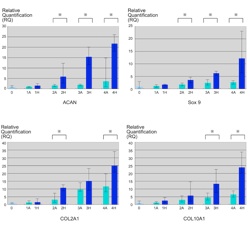
Cartilage regeneration using hydrostatic pressure-loaded adipose-derived stem cells
Chondrogenic differentiation of adipose-derived stem cells in three-dimensional collagen scaffolds is induced by cycles of hydrostatic pressure. Cyclical hydrostatic pressure was effective in increasing the deposition of extracellular matrix and inducing the expression of genes related to chondrogenic differentiation.
Mizuno S, Ogawa R. Using changes in hydrostatic and osmotic pressure to manipulate metabolic function in chondrocytes. Am J Physiol Cell Physiol. 2011 Jun;300(6):C1234-45.
Ogawa R, Mizuno S. Cartilage regeneration using adipose-derived stem cells. Curr Stem Cell Res Ther. 2010 Jun;5(2):129-32.
Ogawa R, Mizuno S, Murphy GF, Orgill DP. The effect of hydrostatic pressure on three-dimensional chondroinduction of human adipose-derived stem cells. Tissue Eng Part A. 2009 Oct;15(10):2937-45.

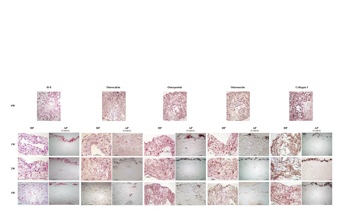
Bone regeneration using hydrostatic pressure-loaded bone marrow-derived mesenchymal stem cells
Hydrostatic pressure increases cellular viability and improves osteogenic differentiation and maturation of mesenchymal stem cells, although at the expense of proliferation and self-renewal. Furthermore, the mechanotransductive molecule, integrin β5, was expressed at high levels after hydrostatic pressure stimulation, a process that may enhance migration, promote differentiation, and inhibit osteoclast maturation during hydrostatic pressure-driven osteogenesis in vitro.
Huang C, Ogawa R. Effect of hydrostatic pressure on bone regeneration using human mesenchymal stem cells.Tissue Eng Part A. 2012 Oct;18(19-20):2106-13.
Huang C, Ogawa R. Mechanotransduction in bone repair and regeneration. FASEB J. 2010 Oct;24(10):3625-32.
Mechanisms Underlying The Developement of Keloids, Hypertrophic Scars, Scar Contractures, and Fibroproliferative Disorders, and The Developement of Prevention and Treatment Strategies: Toward Less-Scar Wound Healing
Until recently, keloid and hypertrophic scars presented surgeons with serious problems in terms of aesthetics. Ugly scars spoil the results of surgery and recur easily, even after revision. However, recent years have seen the steady development of more effective treatment strategies, mainly because of studies that have highlighted the pathophysiological mechanisms underlying the formation of heavy scars.
Keloids and hypertrophic scars develop because of an abnormal wound healing response. Several genetic, systemic, and local factors influence the quality and quantity of such scars. We are the first group to report that SNPs affect the clinical severity of keloids, and that they can be aggravated by hypertension (high blood pressure).
We are particularly interested in mechanical forces, which we believe play an important role in the pathophysiology of keloids and hypertrophic scars. Our laboratory has made some important findings in this area. Keloids appear to show a marked “preference” for particular body locations, and often adopt distinct site-specific shapes. These features are highly dependent on the distribution of mechanical forces in the area. Thus, keloids usually occur at sites that are constantly or frequently subjected to mechanical forces (such as the anterior chest and scapular regions), but seldom occur in areas where stretching/contraction of the skin is rare (such as the parietal region or the anterior lower leg); this even holds true for patients with multiple keloids. Moreover, the typical “butterfly”, “crab’s claw”, or “dumbbell” shapes adopted by keloids appear to be largely determined by the direction of the mechanical forces at and/or around the wound site. Thus, it is suggested that the formation of keloids and hypertrophic scars is due to defects in mechanosignaling pathways. Since these lesions develop in the dermis, we need to fully understand dermal mechanobiology before the pathogenic mechanisms that drive their formation can be fully elucidated.
Dr. Rei Ogawa studied mechanobiology while working with Dr. Dennis Orgill at the Tissue Engineering and Wound Healing Laboratory within the Division of Plastic Surgery at Brigham and Women’s Hospital between 2007 and 2009. Dr. Chenyu Huang is currently working in the same laboratory. Also, Dr. Satoshi Akaishi worked in Dr. Geoffrey Gurtner’s laboratory in the Department of Plastic Surgery at Stanford University between 2010 and 2012. Therefore, our laboratory has close relationships with world-renowned laboratories. Dr. Rei Ogawa also organized the International Scar Meeting in Tokyo (2010) and has been active at various scar meetings and conferences, showing international leadership in the field of wound healing and scarring.
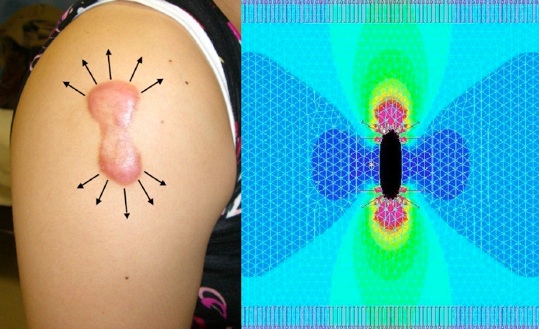
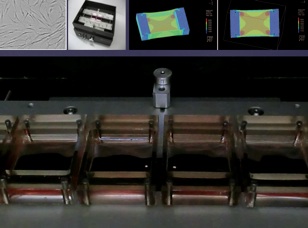
Computer simulation of skin tension around scars
This figure shows a computer simulation of the skin tension around keloids. Stretching the skin longitudinally generates high forces along the edges of the scar (red). This area shows a complete match with the inflamed areas (redness and raised) noted upon clinical examination. Thus, the continuous cycle of stretching generated by body movement has an adverse effect on keloid configuration and inflammation, which cannot be prevented.
Akaishi S, Akimoto M, Ogawa R, Hyakusoku H. The relationship between keloid growth pattern and stretching tension: visual analysis using the finite element method. Ann Plast Surg 60: 445-451, 2008.
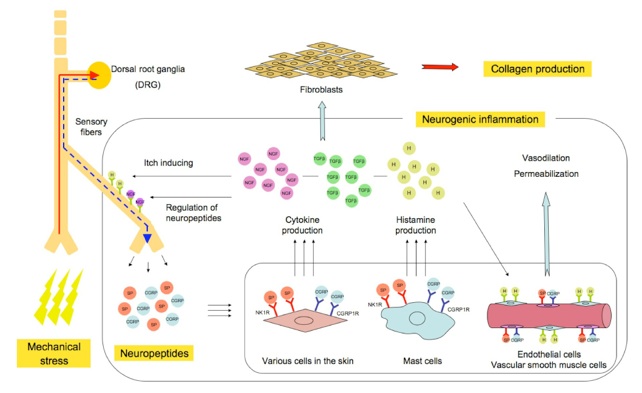
The neurogenic inflammation hypotheses
We also studied the factors that connect mechanical stress with keloid formation. We have developed the so-called "neurogenic inflammation hypothesis", which can be explained as follows: mechanical forces, including skin stretching, stimulate the mechanosensitive nociceptors within the sensory fibers of the skin. The stimulated fibers release neuropeptides, including SP and CGRP, which bind to the receptors SP-NK1R and CGRP-CGRP1R, which are expressed by various cells in the skin. Moreover, histamine release by mast cells is up-regulated. The subsequent activation of endothelial and vascular smooth muscle cells induces vasodilation and blood vessel permeabilization. The production of cytokines, including TGF-β and NGF, by various cells is also stimulated. Neurogenic inflammation and the upregulation of TGF-β secretion then activate fibroblasts via various signaling pathways. Interestingly, once the malignant cycle has started, the overexpression of NGF may induce the hyper-release of neuropeptides from sensory fibers, resulting in the accumulation of neuropeptides even in the absence of mechanical stress.
Akaishi S, Ogawa R, Hyakusoku H. Keloid and hypertrophic scar: neurogenic inflammation hypotheses. Med Hypotheses. 2008;71(1):32-8.
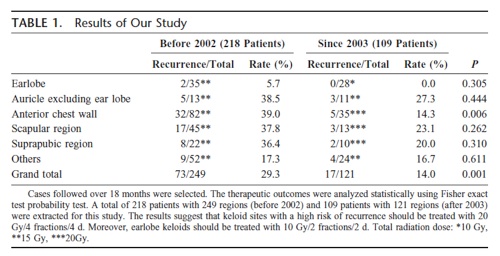
Clinical data regarding keloid treatment at the Nippon Medical School Hospital
We have relied on the knowledge and experience of the staff at our hospital to treat patients with intractable keloids and hypertrophic scars, and we have developed several protocols and treatment strategies. In particular, we have developed safer and more effective protocols for radiation- and laser-based therapies.
Ogawa R, Miyashita T, Hyakusoku H, Akaishi S, Kuribayashi S, Tateno A. Postoperative radiation protocol for keloids and hypertrophic scars: statistical analysis of 370 sites followed for over 18 months. Ann Plast Surg. 2007 Dec;59(6):688-91.
Ogawa R. The most current algorithms for the treatment and prevention of hypertrophic scars and keloids. Plast Reconstr Surg. 2010 Feb;125(2):557-68.
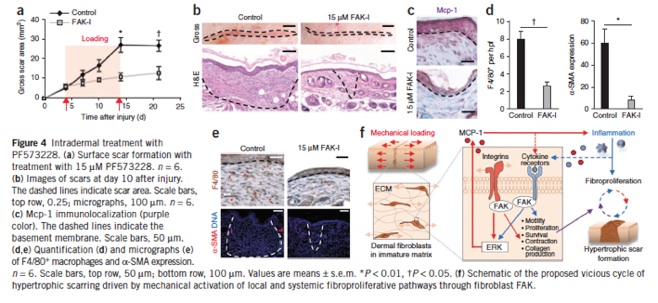
Analysis of focal adhesion kinase in animal models bearing hypertrophic scars
Under the guidance of Dr. Geoffrey Gurtner at Stanford University, Dr. Satoshi Akaishi studied focal adhesion kinase (FAK) in animal models bearing hypertrophic scars. Their studies revealed that FAK is activated by cutaneous injury, and that this process is potentiated by mechanical loading.
Wong VW, Rustad KC, Akaishi S, Sorkin M, Glotzbach JP, Januszyk M, Nelson ER, Levi K, Paterno J, Vial IN, Kuang AA, Longaker MT, Gurtner GC. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med. 2011 Dec 11;18(1):148-52. doi: 10.1038/nm.2574. PubMed PMID: 22157678.
Wong VW, Akaishi S, Longaker MT, Gurtner GC. Pushing back: wound mechanotransduction in repair and regeneration. J Invest Dermatol. 2011 Nov;131(11):2186-96.
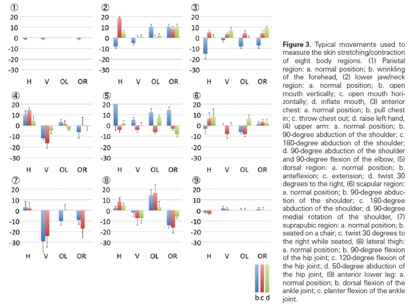
Measurement of skin stretching/contraction rates
Keloids tend to occur at highly mobile sites that are under high tension. We examined whether body surface areas subjected to highly strenuous forces during normal activities showed high rates of keloid generation after wounding. Eight adult Japanese volunteers were enrolled in a study designed to examine skin stretching/contraction rates at nine different sites. Skin stretching/contraction was measured by marking eight points on each region and measuring changes in the location of the marked points after the participants performed typical movements. We mapped the distribution of 1,500 keloids on 483 Japanese patients. We found that the parietal region and anterior lower leg were subjected to the lowest stretching/contraction forces, whereas the suprapubic region experienced the greatest stretching/contraction. With regard to keloid distribution, 733 were present on the anterior chest region (48.9%) and 403 on the scapular regions (26.9%). No keloids were identified on the scalp or anterior lower leg, sites that are rarely subjected to skin stretching/contraction. Therefore, it appears that mechanical forces are an important trigger that drives keloid generation, even in patients who are genetically predisposed to keloids. Thus, mechanotransduction studies are useful for developing new clinical approaches to reducing skin tension around wounds or scars to prevent and/or treat keloids and hypertrophic scars.
Ogawa R, Okai K, Tokumura F, Mori K, Ohmori Y, Huang C, Hyakusoku H, Akaishi S. The relationship between skin stretching/contraction and pathologic scarring: the important role of mechanical forces in keloid generation. Wound Repair Regen. 2012 Mar-Apr;20(2):149-57.
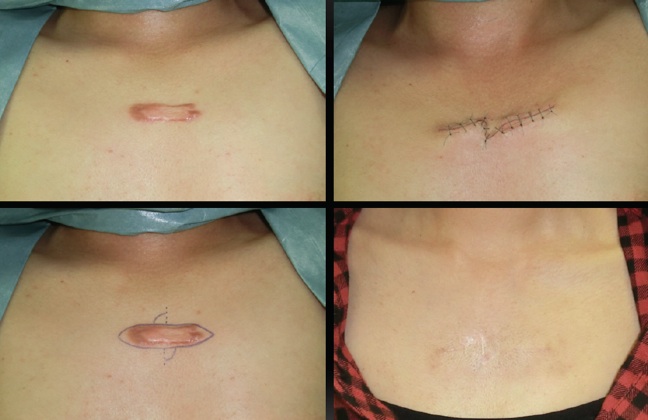
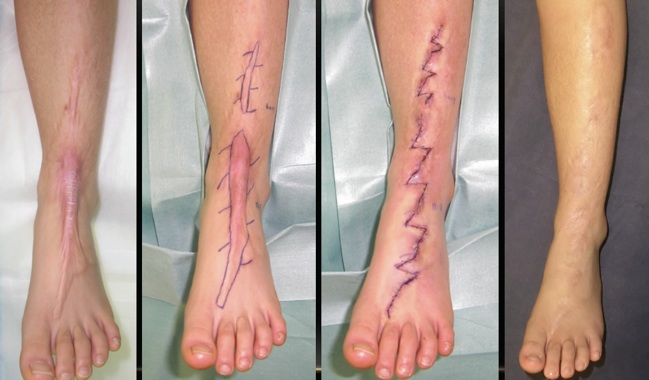
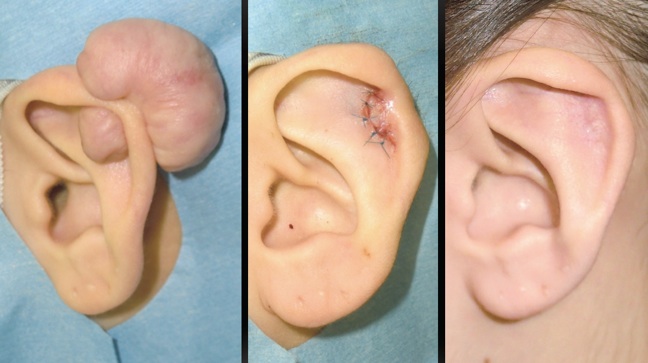
Using mechanotherapy to treat keloid and hypertrophic scars
(Keloid and Hypertrophic Scar Treatments by Mechanotherapy)
Although physicians cannot control patient genetics, it is important to determine whether a patient is predisposed to heavy scarring as this will have a profound effect on perioperative scar management. Thus, the patient should be interviewed to determine whether they have a personal or family history of heavy scarring. If the patient has heavy scars, special attention and care will be needed during the perioperative period.
Unlike genetics, clinicians can control systemic and local factors to some extent. For example, hypertension can be managed by oral drugs and skin tension can be controlled by surgery and taping. Thus, it is easier to plan treatment strategies when the pathophysiology of keloids and hypertrophic scars is taken into consideration. At present, a type of “blind faith” approach based on corticosteroid injections is used to treat heaving scarring; however, this approach is not entirely justified by the outcome. Moreover, many physicians believe that some patients with heavy scars cannot be treated at all. These views are simplistic and out-of-date, and must be reconsidered in the light of new developments.
Current treatment strategies for keloids and hypertrophic scars can be roughly divided into two categories: anti-inflammatory and anti-mechanical forces. Examples of the former include radiation therapy and corticosteroid injections, while examples of the latter include surgery (e.g., z-plasties) and stabilization methods that use silicone gel sheets, surgical tape, and bandages. Categorizing the treatment strategies in this manner may increase our awareness of their intended purposes. As such, we believe that mechanotherapy is the key to preventing and treating heavy scarring.
Orgill DP, Ogawa R. Current methods of burn reconstruction. Plast Reconstr Surg. 2013 May;131(5):827e-36e.
Yagmur C, Guneren E, Kefeli M, Ogawa R. The effect of surgical denervation on prevention of excessive dermal
scarring: a study on rabbit ear hypertrophic scar model. J Plast Reconstr Aesthet Surg. 2011 Oct;64(10):1359-65.
Kuribayashi S, Miyashita T, Ozawa Y, Iwano M, Ogawa R, Akaishi S, Dohi T, Hyakusoku H, Kumita S.
Post-keloidectomy irradiation using high-dose-rate superficial brachytherapy. J Radiat Res. 2011;52(3):365-8.
Akaishi S, Akimoto M, Hyakusoku H, Ogawa R. The tensile reduction effects of silicone gel sheeting.
Plast Reconstr Surg. 2010 Aug;126(2):109e-11e.
Ogawa R. The most current algorithms for the treatment and prevention of hypertrophic scars and keloids.
Plast Reconstr Surg. 2010 Feb;125(2):557-68.
Ogawa R, Miyashita T, Hyakusoku H, Akaishi S, Kuribayashi S, Tateno A. Postoperative radiation protocol for
keloids and hypertrophic scars: statistical analysis of 370 sites followed for over 18 months. Ann Plast Surg.
2007 Dec;59(6):688-91.









