

Scar and Keloid Laboratory
Scar and Keloid Laboratory
News
The research contents have been released on YouTube.
https://www.youtube.com/watch?v=XVkzlBxk8fo&t=16s
Laboratory Mission, Principles, and Agenda
- ✓Elucidating the pathophysiology of scars and keloids
- ✓Develop effective preventive and therapeutic methods for scars and keloids
- ✓Creating a world where there are no patients suffering from scars and keloids
Research
The Scar / Keloid Clinic and Laboratory of Nippon Medical School
Keloids and hypertrophic scars are red scars that grow continuously so that they rise above the surface of the original wound. Keloids tend to be more deleterious than hypertrophic scars because they also relentlessly spread sideways. These pathological scars can be very itchy, painful, and disfiguring. Moreover, if they are located near a joint, they can contract, thereby restricting joint mobility. Common causes include surgery, burns, and trauma but also minor injuries such as highly inflamed acne (known as folliculitis) and piercings. Keloids in particular are especially common in people of Asian and African descent, which suggests that they are at least partly driven by genetics.
Our studies and those of others show that pathological scars are caused by continuous heavy inflammation that starts soon after wounding: while a short early burst of moderate inflammation is needed for normal wound healing, it is ongoing and excessive in pathological scars. The inflammation in turn hyperactivates fibroblasts in the wound/scar. While these cells are important for laying down the extracellular connective fibers and materials (especially collagen) that repair wounded skin, their activity does not abate in pathological scars: instead, the fibroblasts continue to churn out copious amounts of extracellular material long after the wound has closed. This generates large twisted bundles of collagen (called keloidal collagen) that cause the scar to thicken and stiffen.
If left untreated, keloids in particular but also hypertrophic scars near joints can become huge and greatly affect the quality of life of the patient. However, simple surgical removal of these scars is often futile, especially if they are keloids, because they very often grow back in and around the surgical scars, resulting in even worse scars. Moreover, current non-surgical therapies on their own often have weak effects at best, especially with large scars.
To improve the outcomes of patients with pathological scars, we established our Scar/Keloid-specialized Clinic in the Department of Plastic, Reconstructive, and Aesthetic Surgery at Nippon Medical School in Tokyo, Japan in 2005. In the ensuing years, we have treated approximately 2,000 new scar/keloid patients annually. Around the same time, we also consolidated our Scar/Keloid Research Laboratory: therein, we conduct (i) clinical research with the medical data of the thousands of scar patients who have been treated in our Clinic to date and (ii) basic research with keloid tissue and cell culture, genetic analyses, animal models, and computer modelling.
Below, we will describe some of our most seminal studies. Part A discusses the studies that elucidated the various factors and mechanisms that initiate and promote the relentless growth of these quite mysterious scars. Part B describes our studies searching for the most effective clinical techniques. The studies in Parts A and B have together helped us to develop highly effective multi-pronged therapeutic and preventative algorithms for scars of all types, locations, and sizes. As a result, even highly aggressive keloids can now be cured: patients no longer have to endure their scars or undergo multiple rounds of weak and sometimes painful therapies. These studies have also laid the foundation of our current research, which continues to unravel the mechanisms that induce (or prevent) pathological scarring. Our ultimate objective is to manipulate these mechanisms with new drugs and non-surgical techniques so that pathological scars can be treated or even prevented in a rapid and non-invasive manner.
A. Identification of the pathological features of keloids and hypertrophic scars and the factors that promote their growth
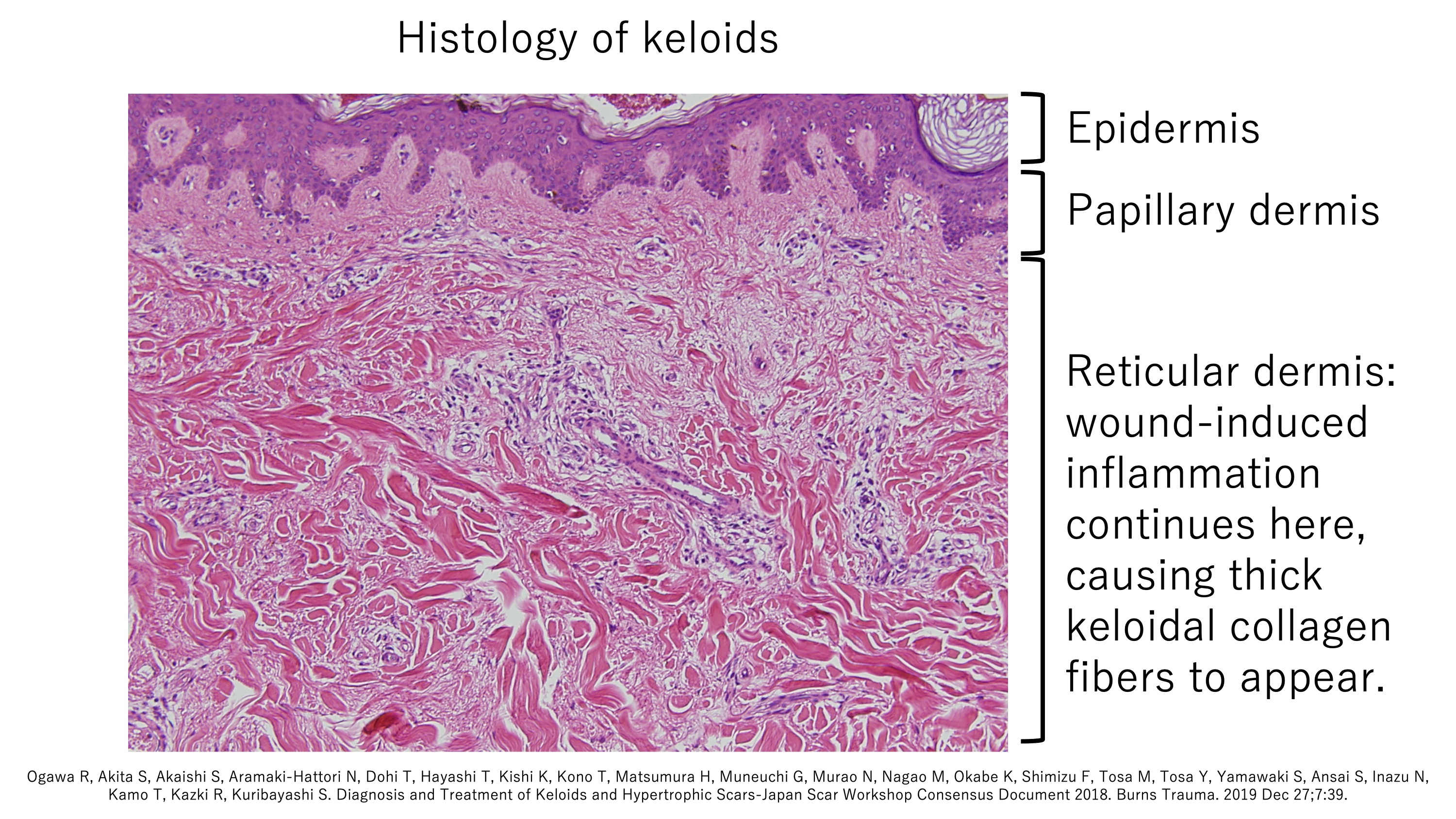
A1. All keloids and hypertrophic scars display chronic inflammation in the reticular layer of the dermis
The skin consists of three layers, namely, the epidermis, papillary dermis, and reticular dermis. Keloids and hypertrophic scars only develop when the dermal reticulum (the deepest layer) is damaged or inflamed. This explains why surgery, which generally involves a full-thickness incision in the skin, is a common cause of pathological scars. For the same reason, ear piercing can cause the ball-like keloids that grow on the ears. Subcutaneous injections that aim to induce local skin inflammation can also generate pathological scars. For example, the BCG vaccination in childhood can cause the dumb-bell-like keloids that run down the upper arm (note that current coronavirus vaccines are delivered in the muscle and thus are very unlikely to induce the dermal inflammation that initiates keloids). Other causes of dermal inflammation can also generate keloids, including highly inflamed acne (folliculitis): this can lead to keloids of the chest, jaw, neck, and shoulders in young people. Keloids and hypertrophic scars can also occasionally arise from shallow abrasions if the wounds become infected and the inflammation spreads to the reticular dermis.
Understanding how keloids and hypertrophic scars start helps us to prevent these scars or catch them while they are small and easier to treat. To this end, we have launched a general information program to educate general practitioners and the public about pathological scars and how to limit them, including thorough cleaning of even minor abrasions and monitoring children for piercing-, vaccine-, and acne-induced keloids.
Reference:https://www.ncbi.nlm.nih.gov/pubmed/28287424
A2. Multiple factors act together to induce the formation and exacerbation of keloids and hypertrophic scars
In sections A3–5, we describe several major risk factors for the development and exacerbation of keloids and hypertrophic scars. They can be broadly categorized as (1) local, (2) genetic, and (3) systemic factors. Our 2020 review also elucidates a fourth category, namely, (4) environmental (lifestyle) factors.
- Local Factors
As indicated in sections A3–4, the most critical local factor is the mechanical force that is exerted on the scar by skin stretching. The impact of these forces is worsened when collagen fibers accumulate locally, as they do in pathological scarring. This is because the collagen stiffens and hardens the scar tissue, preventing the skin from effectively dissipating the tension imposed by body movements. This leads to excessive traction on the surrounding normal skin, which promotes the spread of inflammation into adjacent tissues. It is thought that this mechanism encourages keloids to spread into the surrounding healthy skin.
- Genetic Factors
Section A5d discussed our finding that single nucleotide polymorphisms (SNPs) associate with severe keloids. Other studies have also detected other SNPs that are linked to keloid formation. The notion that inheritable genetic factors can dictate susceptibility to keloids is also supported by the ethic predisposition of Asians and Africans to keloids, and the association of genetic disorders such as Rubinstein-Taybi syndrome and Warburg-Cinotti syndrome with keloids.
- Systemic Factors
In our 2020 review, we discuss in particular the systemic factors that may promote pathological scarring. As described in Section A5, these factors include female sex hormones, circulating inflammatory factors, hypertension, and youth.
- Environmental (Lifestyle) Factors
Several environmental (lifestyle) factors can also worsen keloids and hypertrophic scars. They include diet, psychological stress, weight gain, sleep deprivation, smoking, strenuous physical activity (e.g. manual labor or gym training), and behaviors that cause excessive blood flow. The latter include heavy alcohol consumption, excessive intake of spicy foods, and prolonged bathing. All of these environmental factors are technically also systemic factors but they form a separate subcategory because they are more modifiable than the other systemic factors.
Given this plethora of risk factors, it is likely that individuals with multiple risk factors are more prone to pathological scarring, may develop particularly severe scars, and may find their scars are resistant to treatment. By contrast, those with fewer risk factors are more likely to respond well to therapy. These principles have been incorporated in our Japan Scar Workshop Scale, which helps clinicians distinguish hypertrophic scars, which are relatively responsive to treatment, from keloids, which can be highly refractrory to treatment (see section B2).
Reference:https://pubmed.ncbi.nlm.nih.gov/32856341/
A3. Lines of evidence support the importance of local mechanical stimulation in driving pathological scarring
Several studies from our group further support the notion that local tension on wounds/scars is a potent stimulant of pathological scarring.
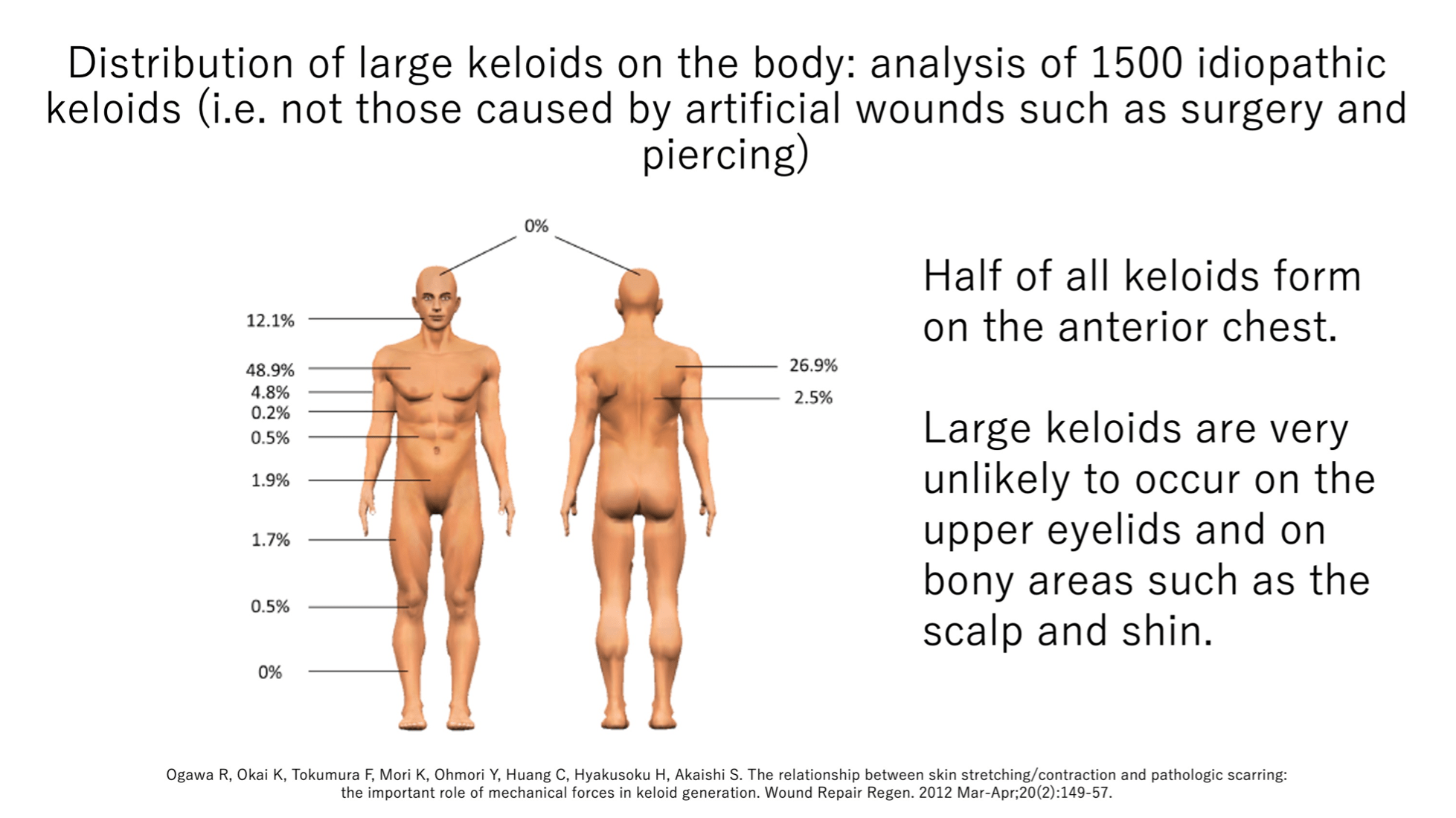
A3a. Half of all idiopathic keloids occur on the anterior chest while none occur in bony regions
Our analysis of data from 1,500 patients visiting our Scar/Keloid Clinic showed that in the Japanese population, 49% of large idiopathic keloids (i.e. those not caused by surgery or piercing) occur on the anterior chest.
An analysis of the causes showed that the main cause of keloids on this region was folliculitis. Thus, children and young people should be monitored carefully if they have anterior chest folliculitis.
This analysis also showed that keloids are extremely rare on regions with bone immediately under the skin, namely, the scalp and shin. They also never occur on the upper eyelids.
These observations suggest that wounds on the anterior chest should be followed closely whereas injuries or surgery on non-susceptible areas require less attention.
Reference:https://www.ncbi.nlm.nih.gov/pubmed/22332721
A3b. Keloids, hypertrophic scars, and scar contractures tend to occur in areas that move frequently
The distinct keloid localization pattern described above may reflect the fact that the keloid-prone body regions undergo regular skin stretching due to body movements whereas the non-susceptible scalp and shin do not. With regard to the upper eyelid, its skin sags naturally and thus eye closing and opening does not induce stretching tension. These findings suggest that local stretching mechanical force can promote pathological scar growth.
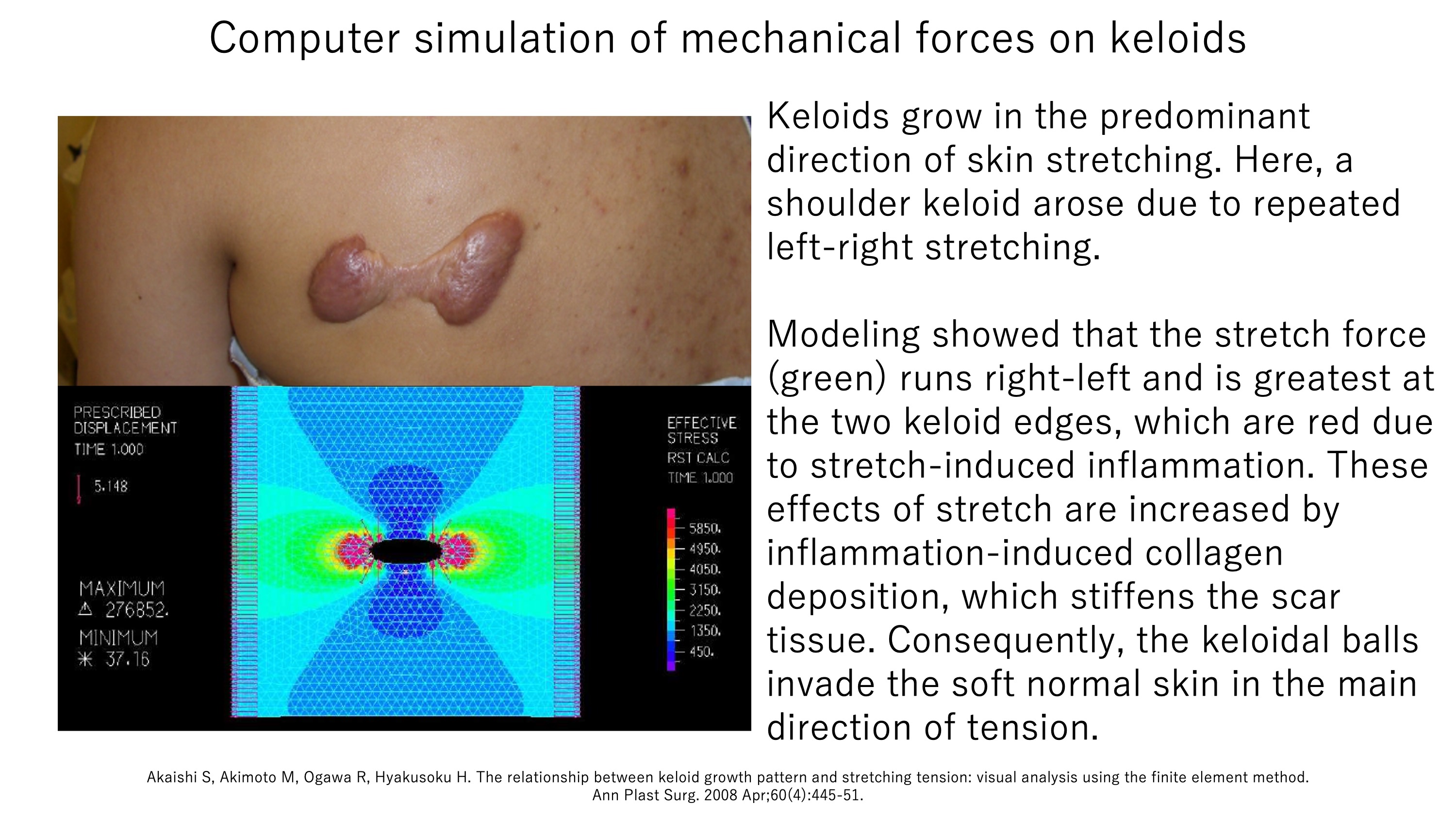
This notion is supported by two other findings. First, joints are highly prone to contractile hypertrophic scars: while hypertrophic scars generally subside spontaneously after a few years, they often will not do so if they are near a joint.Instead, these scars will continue growing to form contractile scars that impede joint movement.Second, keloids tend to adopt region-specific shapes that directly match the predominant directions of skin tension. For example, keloids on the anterior chest (which is constantly undergoing powerful left-right stretching due to arm movements) often develop a crab’s claw shape. Another example is shown by our computer simulation analysis, which modeled the mechanical force on a left-right-running dumb-bell-shaped shoulder keloid. This analysis showed that (i) the predominant stretching tension on the shoulder also ran in the left-right direction and (ii) while the normal skin bore moderate tension (green), the highly inflamed leading edges of the keloid balls exhibited enormous tension (red). Thus, it may be that stretching tension increased the inflammation at the leading edges of the two keloid balls, causing further collagen deposition in these regions and promoting the steady invasion of the keloid balls into the soft surrounding normal skin. This process may be exacerbated by the stiffening effect of the new collagen, which amplifies the force on the leading edges and leads to a vicious circle of mechanical force-driven inflammation and scar growth/invasion.
These observations explain for example why training in the gym can cause keloids on the chest or abdomen to deteriorate and why minor wounds near a joint can result in severe scar contractures. This knowledge has helped us to identify the body areas that are particularly at risk of pathological scarring and therefore require close attention during and after surgery.
Reference:https://www.ncbi.nlm.nih.gov/pubmed/18362577
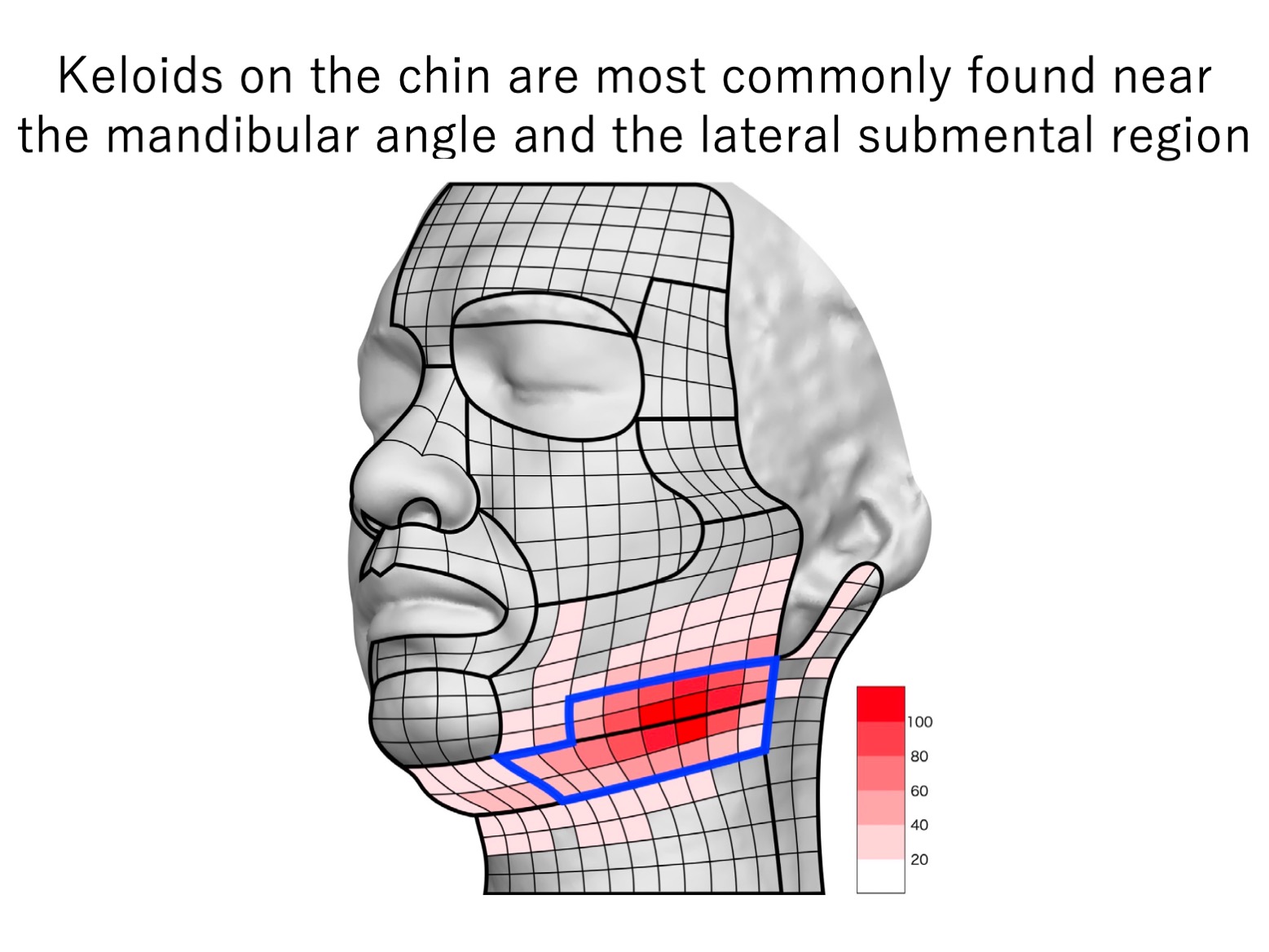
A3c. Chin (mandibular) keloids specifically develop in areas that are prone to stretching tension
Our analysis of 113 patients with keloids on the face and neck showed that the keloids occurred most frequently near the mandibular angle (41.3%) and the lateral submental region (20.0%). Significantly, these are the facial regions that are particularly prone to stretching tension from neck movements.
It is often thought that mandibular keloids are caused by acne but our findings did not support this: the high-frequency sites of mandibular keloids did not correlate consistently with areas of high sebaceous-gland density or regions that are prone to acne.
Thus, this study supports the notion that mechanical stimuli due to body movements promote the development of keloids and hypertrophic scars.
Reference:https://pubmed.ncbi.nlm.nih.gov/38602106/
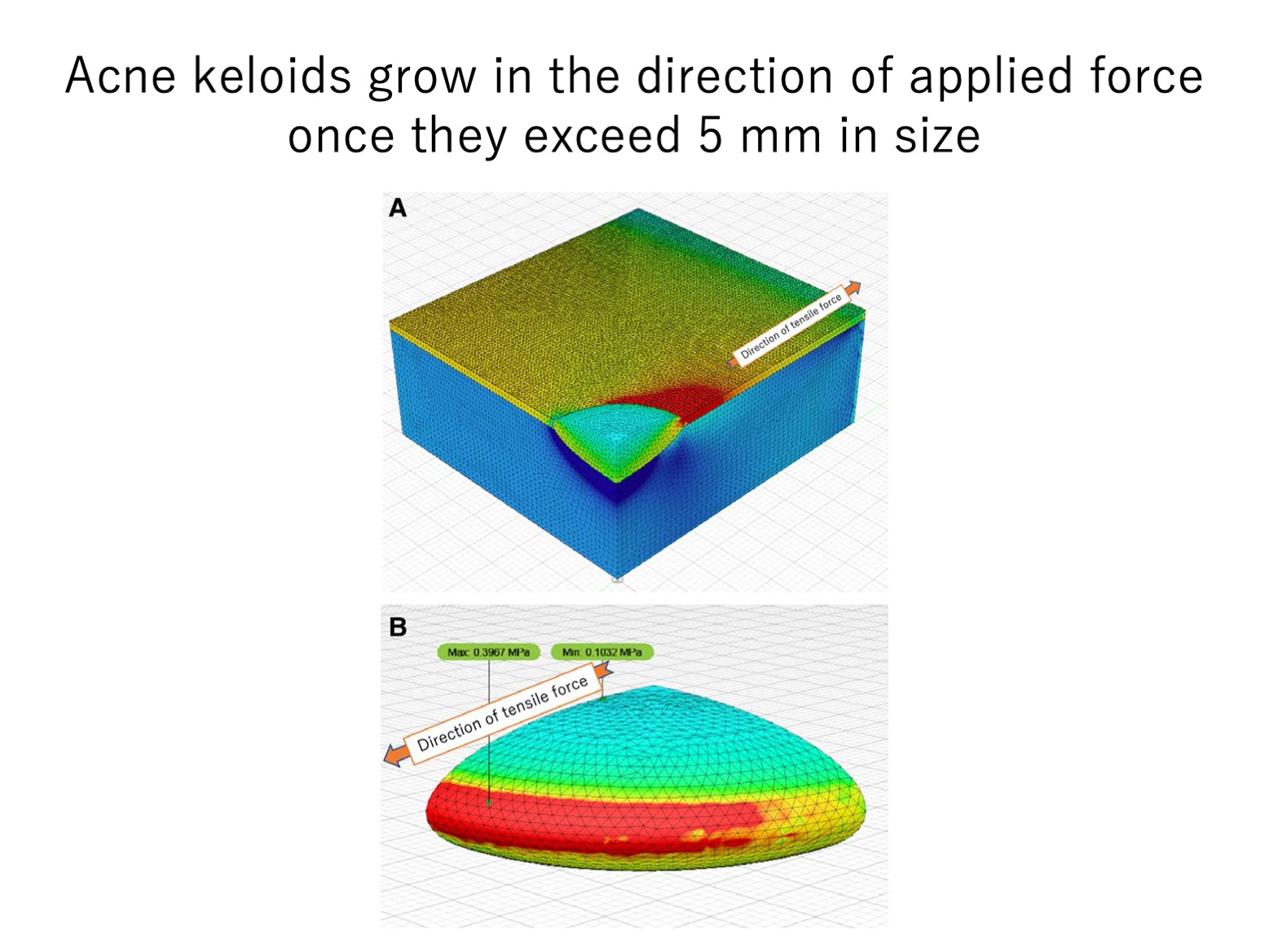
A3d. Acne keloids larger than 5 mm change from a round shape to a spindle shape due to tension
Acne is well known to trigger keloids, likely because it induces dermal inflammation. Since acne wounds are typically round, we asked whether acne keloids change shape as they grow. Indeed, our statistical analysis of the dimensions of 220 acne keloids showed that the keloids remained round until they became 5 mm in diameter: thereafter, they elongated rapidly, likely due to the effect of mechanical tension on the scars caused by body movements.
We next conducted finite element analysis to determine how the distribution of mechanical stress on acne keloids changes as the keloids grow. Thus, we generated computer-simulated acne keloids, placed them in simulated skin, and subjected them to unidirectional traction. We found that when the simulated acne keloids achieved a diameter of 4–5 mm, they expanded beyond the dermis and the tractional stress shifted from the keloid core to the healthy dermis next to the leading keloid edge.
These findings support the notion that keloids spread into the healthy surrounding tissue because the presence of the hard lesion concentrates tractional tension onto the surrounding skin near the lesion margin, thus inducing inflammation in the adjacent skin. This notion was further supported by our microscopic analyses of acne keloid samples, which showed perturbation of the extracellular matrix in the healthy skin near the leading keloid edge.
These findings are of clinical importance: they suggest that keloids should be treated while they are still small and have not yet distorted the mechanical forces on the surrounding skin.
Reference:https://pubmed.ncbi.nlm.nih.gov/39712380/
A4. Other risk factors for pathological scarring
The studies described above suggest that mechanical tension on wound/scar edges can induce and worsen pathological scarring. Our studies described below have identified a number of other risk factors for pathological scarring or its worsening. This information may help us to identify patients most at risk of heavy or worsening scarring as well as point to biological pathways that could be targeted therapeutically by drugs or non-invasive treatments.
A4a. Female hormones may promote susceptibility to keloids
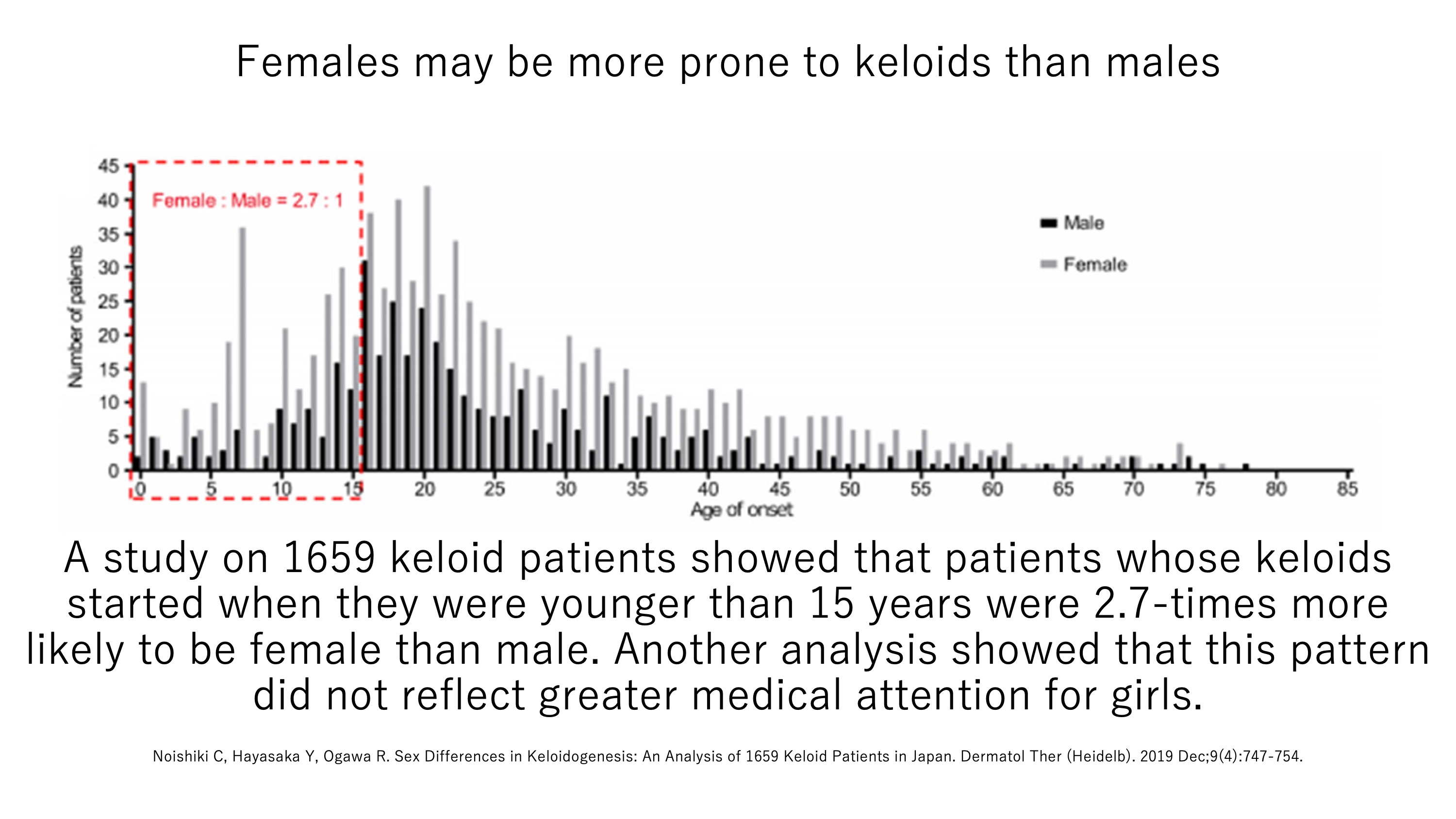
It is well-known that women are more likely to be treated for keloids than men. Indeed, our analysis of data from 1,659 patients who visited our Scar/Keloid Clinic showed that keloid patients are 2–3 times more likely to be female than male. The prevailing view in the field is that this reflects social factors (e.g. women are more likely than men to seek medical attention for unappealing scars) rather than a genuine predisposition of females to keloids. However, when we analyzed the data of people who developed keloids in childhood, we observed that girls were three times more likely than boys to develop keloids and that this did not reflect greater medical attention. The possibility that female hormones could promote keloid formation is currently being researched further.
Reference:https://www.ncbi.nlm.nih.gov/pubmed/31586308
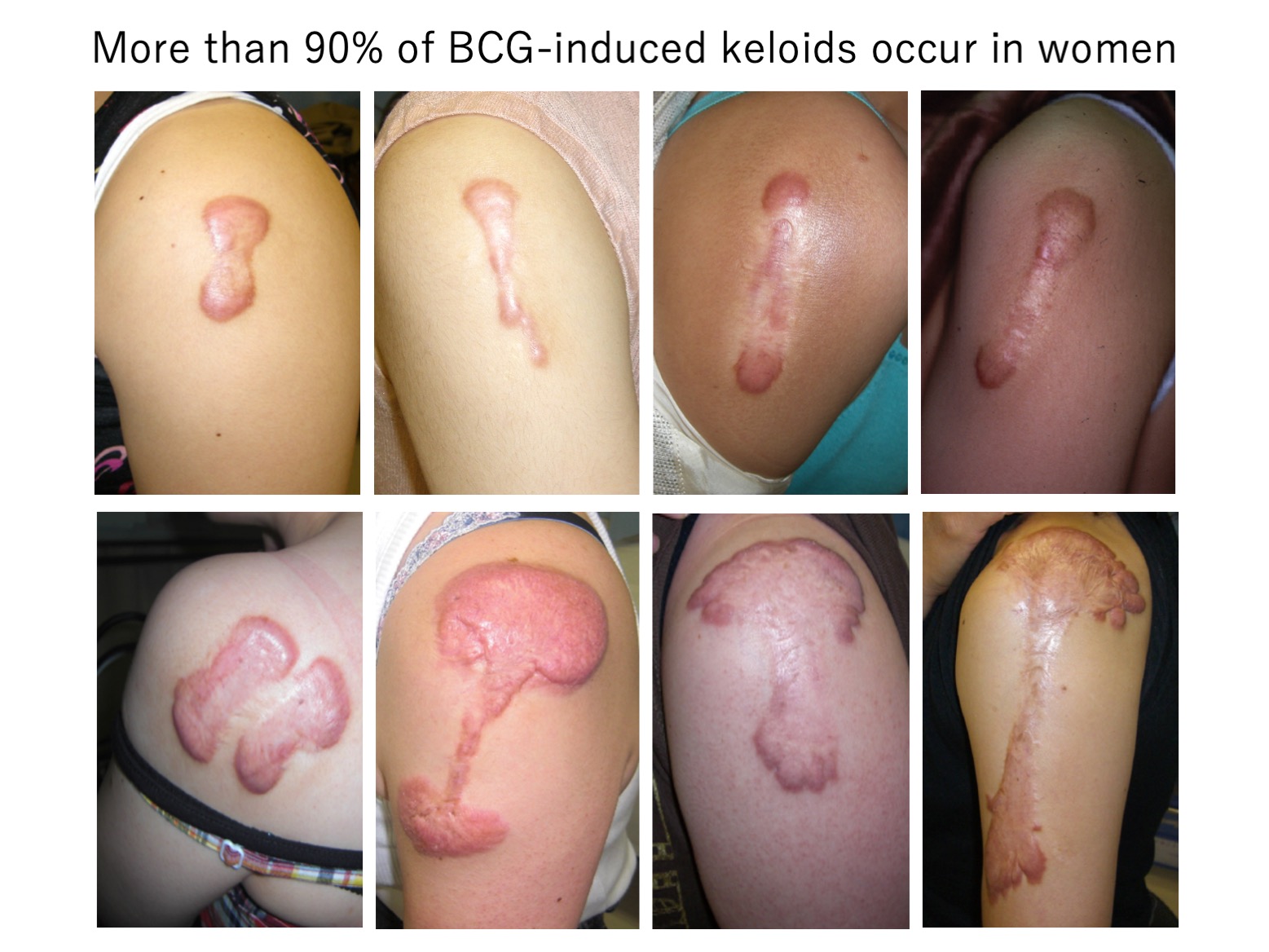
A4b. Keloids caused by BCG vaccination occur predominantly in women
A classical keloid is that on the shoulder: this dumb-bell-shaped scar is caused by the BCG vaccination, which used to be administered twice: once in infancy and again in childhood. The shoulder keloids generally arose after the second dose. It is possible that the mechanical tension on the shoulder skin caused by the rapid growth of the child contributes to the formation of these keloids.
Interestingly, our statistical analysis of 1443 keloid cases at Nippon Medical School Hospital showed that despite the fact that both males and females receive the BCG vaccine, the vast majority (92.9%) of keloids caused by the BCG vaccination occur in female patients.
This striking finding strongly supports the notion that female sex is a risk factor for keloids, It is possible that female hormones and their attendant effects on immune responses are responsible. However, it is also possible differences in body size and physical activity levels at the time of vaccination contribute to this predilection. Further investigation is needed to clarify these mechanisms.
With regard to the BCG vaccination, the vaccination schedule was changed to a single dose in infancy after the 2004 revision of the Tuberculosis Prevention Act. Consequently, keloid development due to BCG vaccination has become rare in individuals born after 2004. It is likely that the risk of BCG-induced keloids is now minimal, except in individuals with a strong predisposition to keloids.
Reference:https://pubmed.ncbi.nlm.nih.gov/36952124/
A4c. Inflammatory cytokines exacerbate keloids
Inflammation in general is mediated in large part by small molecules called cytokines. We recently reported the details of an interesting case of a patient who developed Castleman’s disease, which is characterized by abnormal levels in the blood of a cytokine called interleukin-6. The patient had ear keloids that were small and relatively inactive until she developed Castleman’s disease: at that point, the keloids grew very rapidly and required surgery. This case suggests that inflammatory cytokines can worsen keloids. This supports the notion that inflammation plays a key role in both keloid formation and growth.
Reference:Reference:https://www.ncbi.nlm.nih.gov/pubmed/28607862/
A4d. Single nucleotide polymorphisms associate with severe keloids
The predominance of keloids in Asians and Africans suggests that these scars are partly driven by genetics. This is supported by our analysis of cells in the blood of patients treated at our Scar/Keloid Clinic, which showed that patients with severe keloids have a small mutation in the NEDD4 gene (known as rs8032158). We are conducting further studies to determine how this mutation could promote severe keloid growth.
Reference:https://www.ncbi.nlm.nih.gov/pubmed/24496234
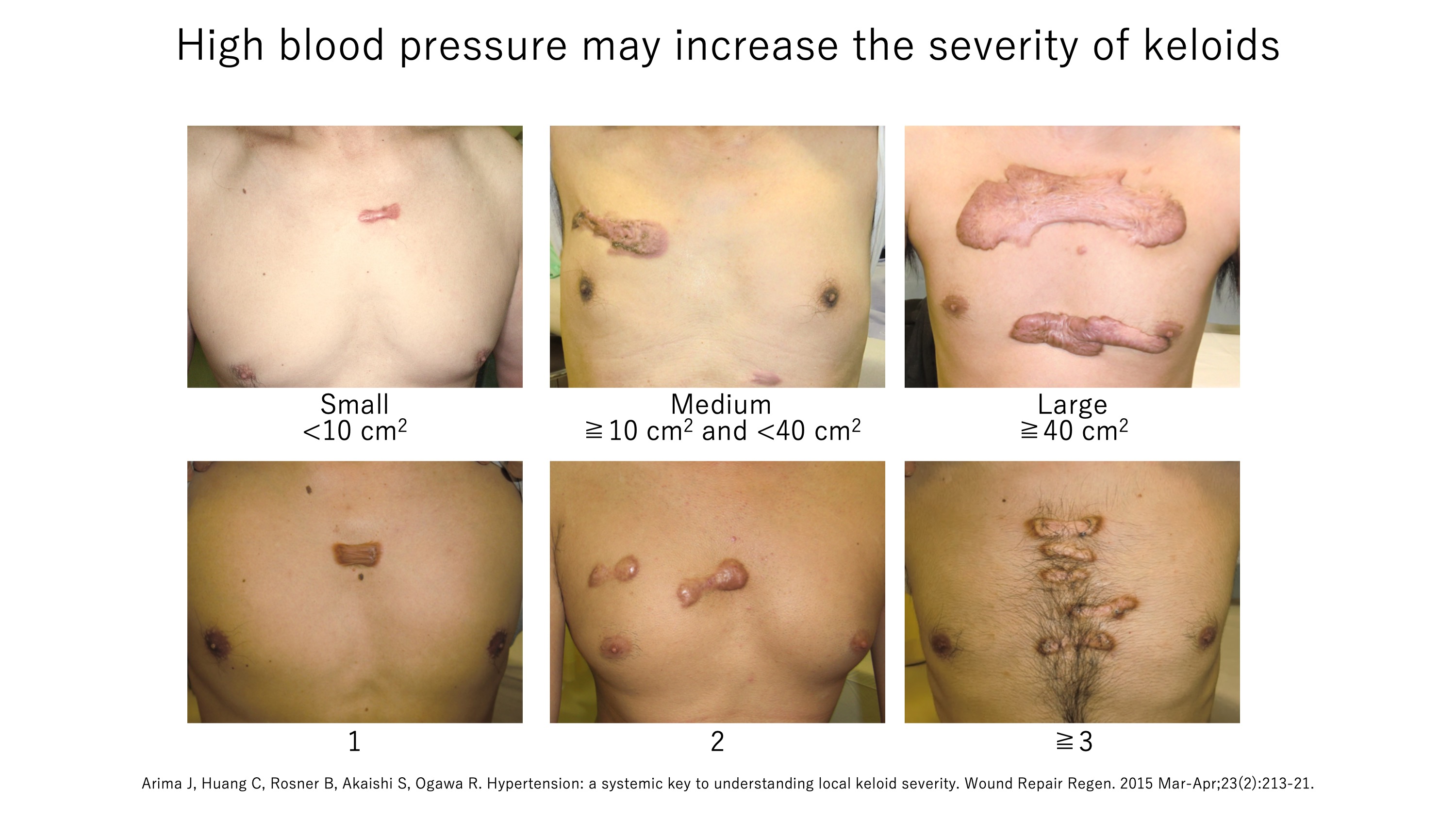
A4e. High blood pressure worsens keloids
Our analysis of the data of patients treated at our Scar/Keloid Clinic showed that patients with severe keloids (i.e. many or very large keloids) were much more likely to have hypertension than patients with milder keloids. We believe that high blood pressure may worsen keloids because it adds to the mechanical tension on the endothelial cells.
Reference:https://www.ncbi.nlm.nih.gov/pubmed/25728259
A4f. Severe arteriosclerosis associates with lower susceptibility to keloids and hypertrophic scars
Given that the chest skin is subject to considerable stretching tension due to upper-arm movements, it is the most keloid-prone site and surgical scars such as median sternotomy often develop into keloids or hypertrophic scars. To determine the risk factors for such scars, we surveyed 328 Japanese patients who underwent median sternotomy. Of these patients, 60% developed pathological scars. This confirms the proneness of the chest to these scars.
Compared to the patients with mature scars, the patients with pathological scars were younger and had less blood vessel narrowing due to arteriosclerosis. The association between younger age and pathological scars may reflect the fact that these scars are driven by chronic inflammation and younger people have more vigorous immune systems than older people. The association between less arteriosclerosis and pathological scarring may reflect the fact that in arteriosclerosis, the blood vessels bear many macrophages that degrade collagen: since pathological-scar growth is caused by collagen accumulation, the collagen-degrading activity of these macrophages in patients with atherosclerosis may help prevent the formation of keloids and hypertrophic scars.
Thus, younger age is a risk factor for pathological scarring while atherosclerosis is a protective factor.
Reference:https://pubmed.ncbi.nlm.nih.gov/35787599/
A5. Abnormal blood vessels may play a key role in keloid formation and growth
Our studies described above indicate that local mechanical tension can provoke or worsen wound/scar inflammation and excessive collagen deposition. How mechanical tension promotes pathological scarring is not yet clear but we speculate that abnormal blood vessel function may be a key mediator.
A5a. Endothelial dysfunction may play a key role in keloid formation and growth
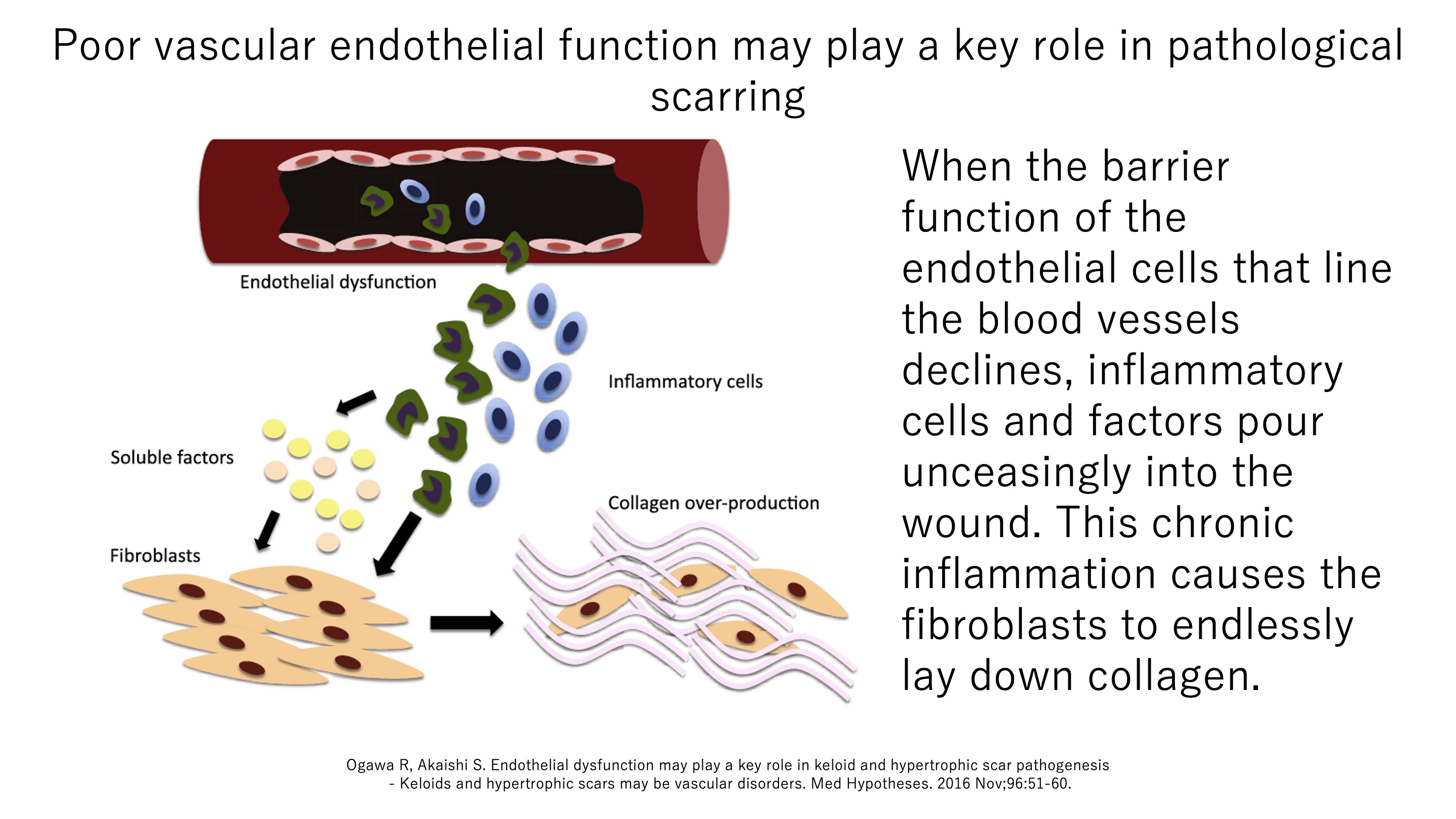
An important role of blood vessels is to act as a dynamic barrier between tissues and the circulation. Thus, the endothelial cells that line the blood vessels usually adhere tightly to each other, thereby preventing cells and large molecules in the circulation from entering the tissues. However, if a tissue has been wounded, it sends signals to the nearby blood vessels, which causes their endothelial cells to pull away from each other, thus allowing cleaning and reparative inflammatory cells and molecules to enter. This process is called vascular permeabilization. Endothelial cells are known to be exquisitely sensitive to mechanical forces. We speculate that if these cells are subjected to strong, repetitive, and unending pulling, the vascular permeability after wounding does not return to normal. Consequently, inflammatory cells and factors continue to flow in and activate fibroblasts, which cannot stop depositing collagen. Several of our studies support this scenario, as follows.
Reference:https://www.ncbi.nlm.nih.gov/pubmed/29240273
A5b. Keloids associate with decreased vascular endothelial cell function
When we analyzed patients with and without keloids, we observed that keloid patients had significantly poorer vascular endothelial function than the control patients. Notably, this was particularly true for patients whose keloids started before adolescence or at an older age. We speculate that the young-onset patients have genetic factors that predispose them to poor endothelial cell function at an early age while the older-onset patients have acquired diseases such as hypertension and diabetes that cause endothelial cell functions to deteriorate.
These findings suggest that drugs (e.g. anti-hypertensive medications) or treatments that improve endothelial function could (i) ameliorate or prevent pathological scarring and (ii) result in scar-less wound healing.
Reference:https://pubmed.ncbi.nlm.nih.gov/28403507/
A5c. Histology of keloids show a close spatial and temporal relationship between deranged blood vessels, fibroblasts, and keloidal collagen production
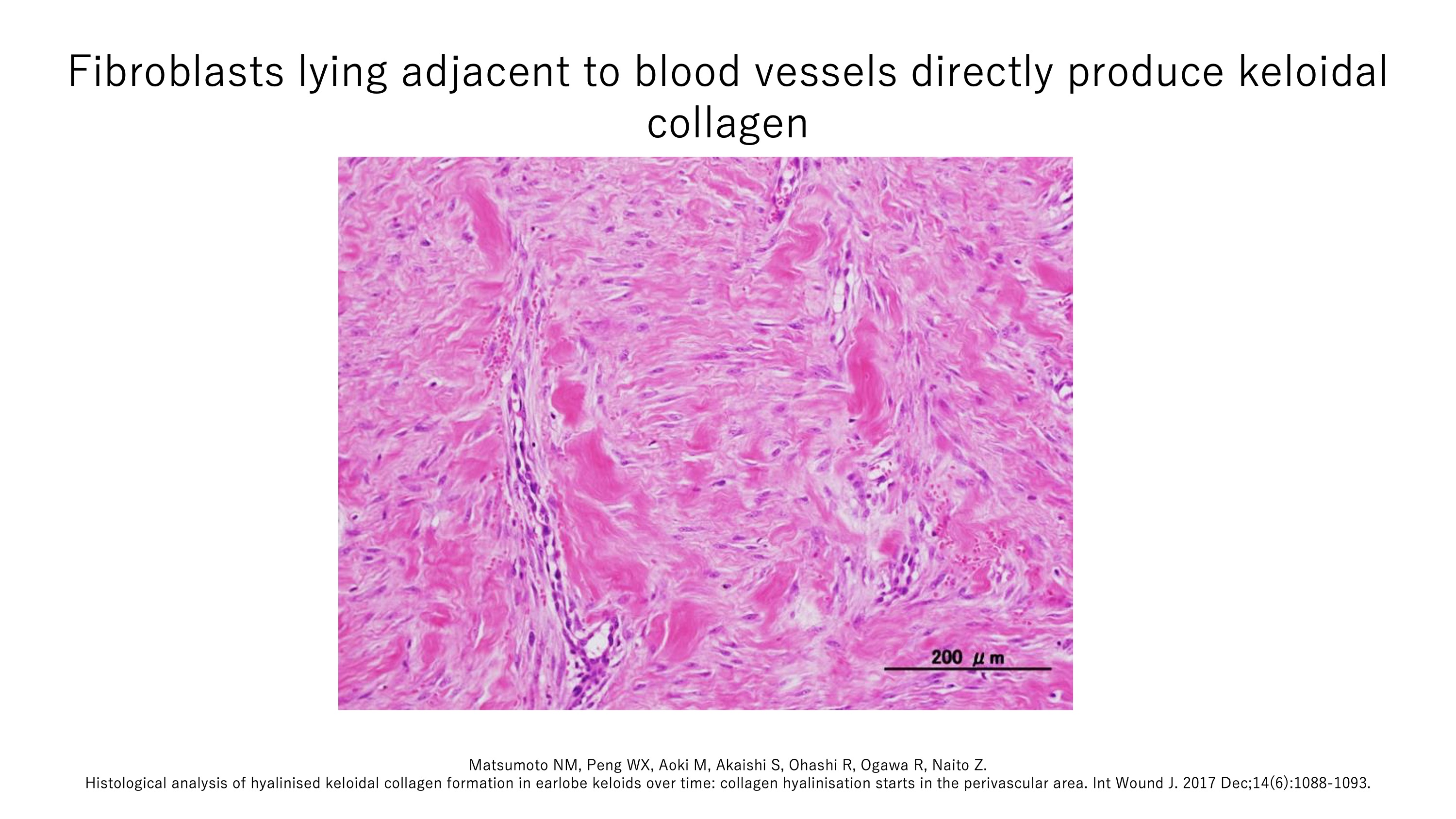
When keloids are cut into slices and examined under the microscope (a procedure known as histology), their reticular dermis is observed to contain so-called keloidal collagen, which is characterized by abnormally thick, twisting, translucent ropes of fused collagen fibers. Keloidal collagen is responsible for the bulkiness of keloids. The prevailing view in the field is that these collagen structures are the end-result of inflammation, which has caused the collagen to degrade. However, keloidal collagen is not seen in other diseases. Our recent histology studies showed that in fact (i) keloidal collagen is directly produced by fibroblasts, (ii) these fibroblasts all lie adjacent to the blood vessels, (iii) the blood vessels are abnormal (often occluded, narrowed, or disrupted and appear to spill cells and cell fragments into the reticular dermis), and (iv) keloidal collagen starts being produced very soon after keloid onset.
These studies support the notion that impaired blood vessels allow ongoing local inflammation, which causes nearby fibroblasts to directly produce keloidal collagen, which in turn induces keloids to expand beyond the original wound boundaries.
Reference:https://www.ncbi.nlm.nih.gov/pubmed/28467018
A5d. Keloid patients are normal in terms of blood vessel production
The redness of keloids is due to the abnormal abundance of blood vessels. New blood vessels are produced by precursor endothelial cells that circulate in the blood. Together with the fact that keloid is partly a genetic disease, our suspicion that abnormal blood vessel functions may mediate keloid development led us to ask whether keloid patients have fundamental defects in new blood vessel formation. However, we found that patients with and without keloids did not differ in terms of how well their precursor endothelial cells produced blood vessels under culture conditions. Thus, the excessive production of blood vessels in keloids is due to local conditions.
Reference:https://www.ncbi.nlm.nih.gov/pubmed/31290139
A5e. The vascular basement membranes in keloids are extremely fragmented
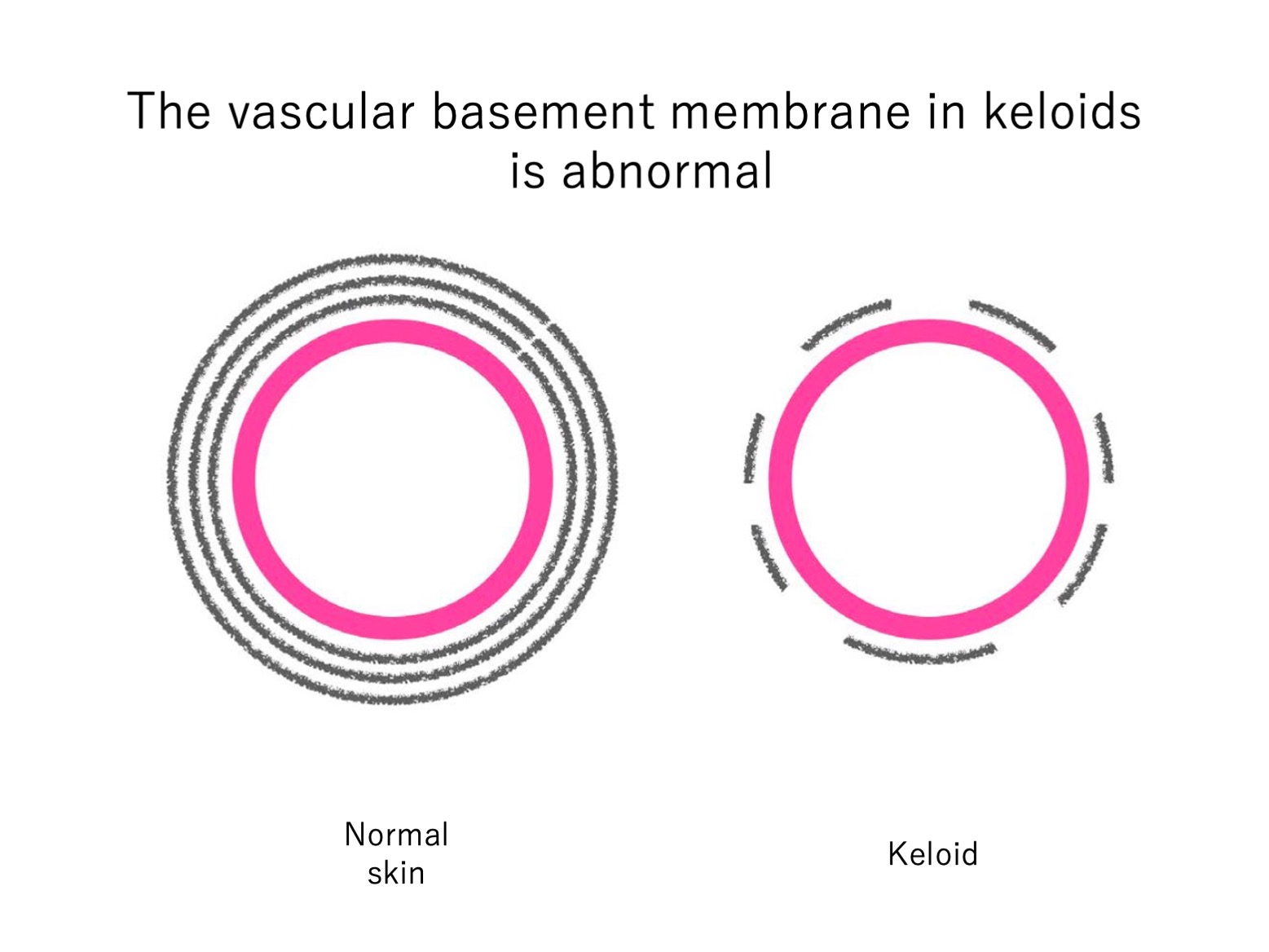
To test whether vascular permeability is increased in keloids, we subjected the capillaries in keloids to electron microscopic analysis. We focused in particular on the vascular basement membranes (VBMs) because the endothelial cells that line skin capillaries are attached to the VBM and the VBM potently regulates vascular permeability. Consequently, any degeneration of the VBM can permit immune cells and inflammatory factors to infiltrate into the interstitial tissue around the blood vessel.
We found that compared to the capillaries in the nearby normal skin, the keloid capillaries had significantly thinner and more fragmented VBMs, and these VBMs bore fewer layers. Thus, keloids associate with disrupted VBMs that could promote vascular permeability. Whether this abnormality is a cause or consequence of keloid formation remains an important subject for future research.
Reference:https://pubmed.ncbi.nlm.nih.gov/39717721/
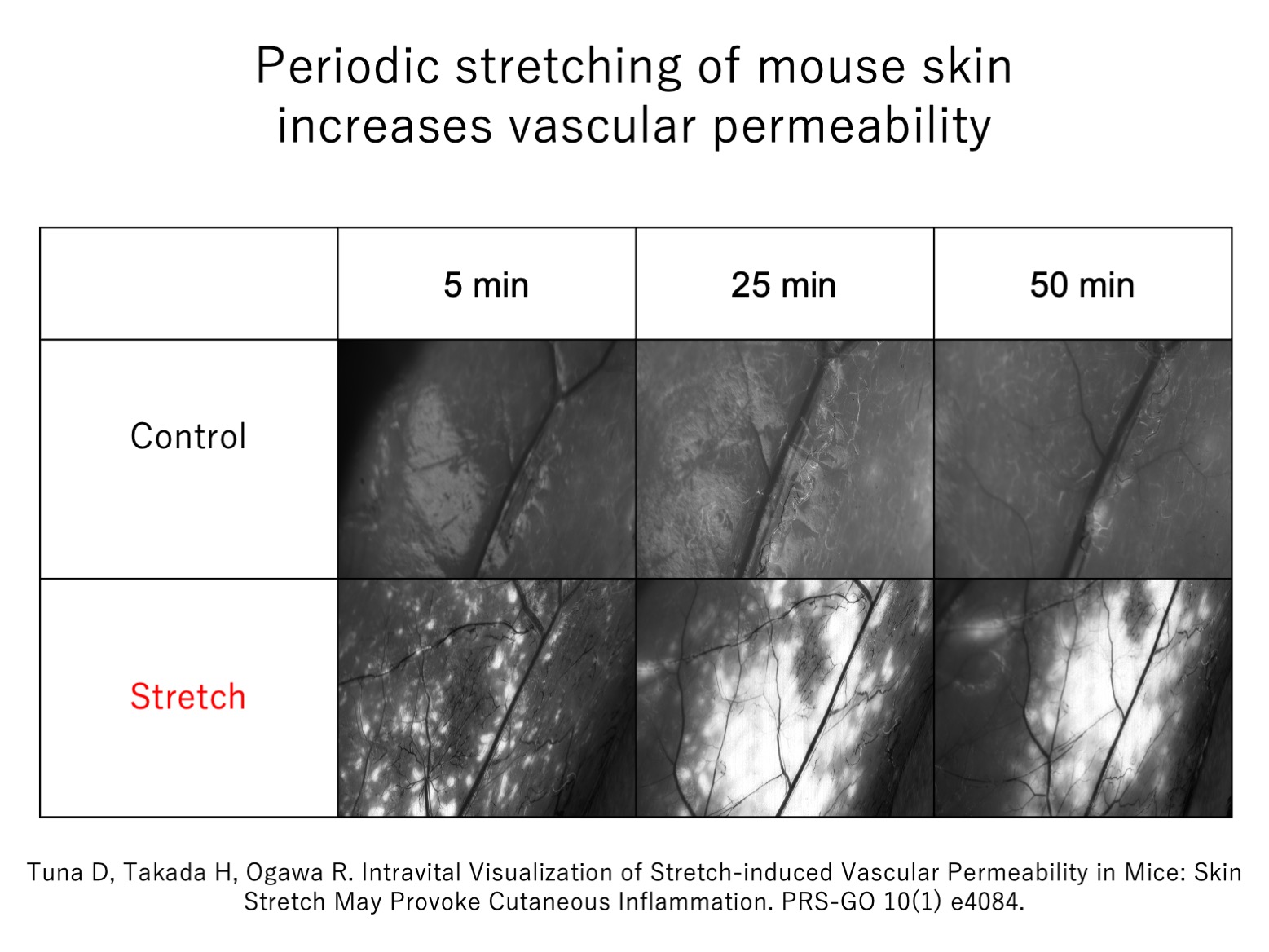
A5f. Vascular permeability increases when the skin is subjected to tension
We hypothesize that mechanical tension promotes vascular permeability, which permits the influx of immune factors in the circulation: in keloids, this process is exaggerated and/or prolonged, leading to the inflammation that drives keloids. To test whether mechanical tension indeed increases vascular permeability in the skin, we subjected flaps of skin on anesthetized mice to 20% cyclic stretching, after which a fluorescent marker was injected into the blood stream. We found that the stretching increased local vascular permeability as strongly as the known vasodilator histamine.
Thus, our study supports the notion that mechanical tension induces pathological scarring by increasing vascular permeability, which allows the influx of inflammatory factors from the circulation.
Our study is also consistent with observations that cyclic movements such as pedaling a bicycle or performing repetitive abdominal or upper-arm movements can increase vascular permeability in abdominal/chest wounds.
Reference:https://pubmed.ncbi.nlm.nih.gov/35186636/
A5g. Keloidal collagen may be produced directly by αSMA-positive cells
Following on from our histological analyses of keloids in section A9d, we examined the cells that produce keloidal collagen in keloids more closely. To this end, we subjected keloids to immunohistochemistry and electron microscopy.
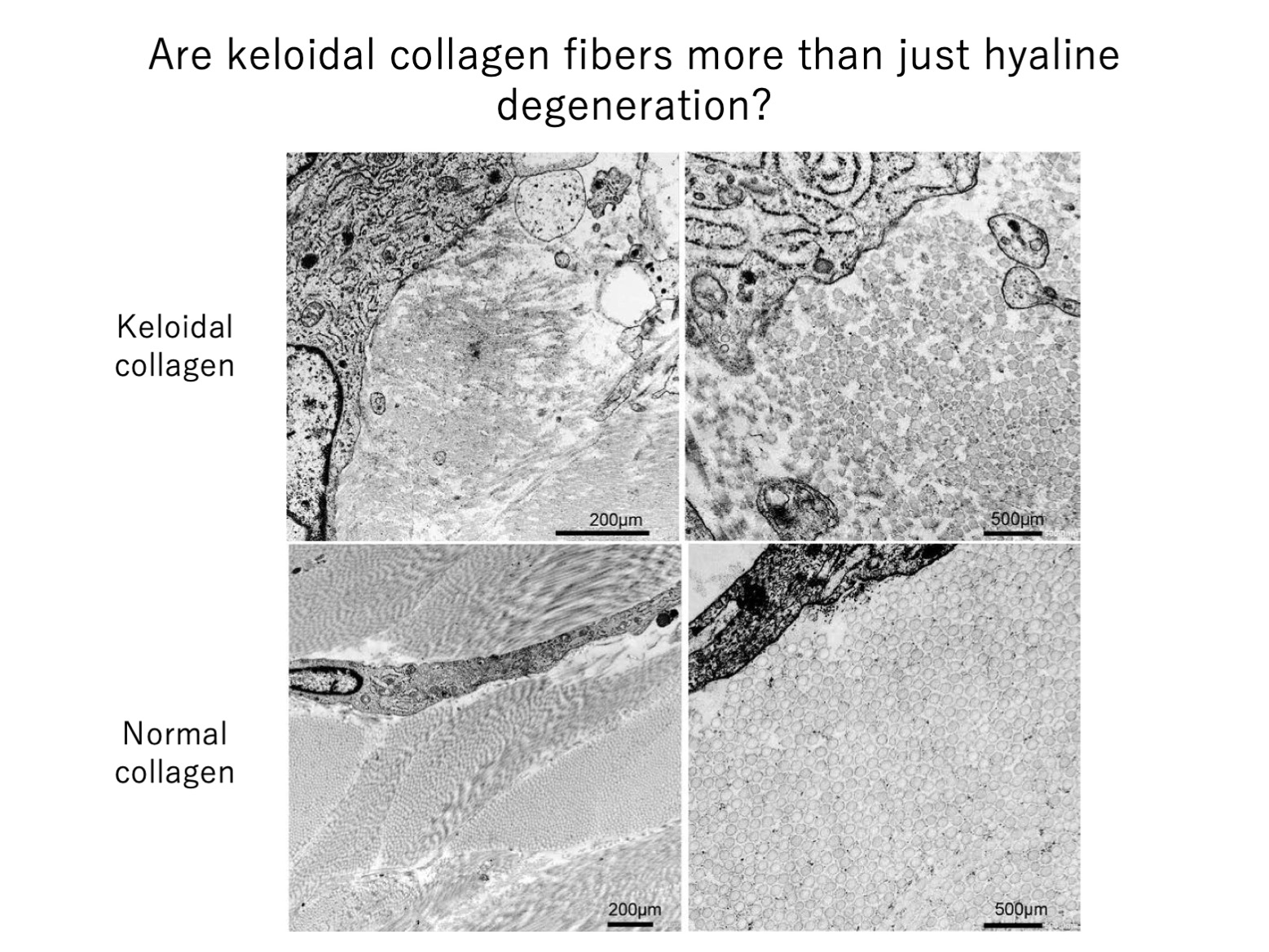
We first found that the keloidal collagen fibers consistently lie directly adjacent to not only blood vessels but also spindle-shaped αSMA-positive cells. αSMA is a marker of smooth muscle cells but is also produced by fibroblasts that have differentiated into mesenchymal cells. These cells behave very differently to classical fibroblasts: they produce very high levels of collagen, respond strongly to mechanical tension, secrete many inflammatory factors, and can powerfully contract the extracellular matrix, thus pulling on other cells. They likely contribute to keloid growth. Thus, our study showed that keloidal collagen is produced directly by vascular smooth muscle cells and/or myofibroblasts that lie near blood vessels. This highlights the importance of the blood vessels in keloid growth. Further research is needed to identify the origin of the keloidal collagen-producing cells.
Our electron microscopy analyses also showed that the collagen fibers within keloidal collagen were generally thin, had varying diameters, and were disorganized, and that these characteristics are particularly pronounced in the collagen fibers near the spindle-shaped cells. This supports the notion that keloidal collagen is directly produced by cells rather than arising from hyaline degeneration of existing collagen fibers.
Reference:https://pubmed.ncbi.nlm.nih.gov/37051211/
A5h. Keloids exhibit congestion
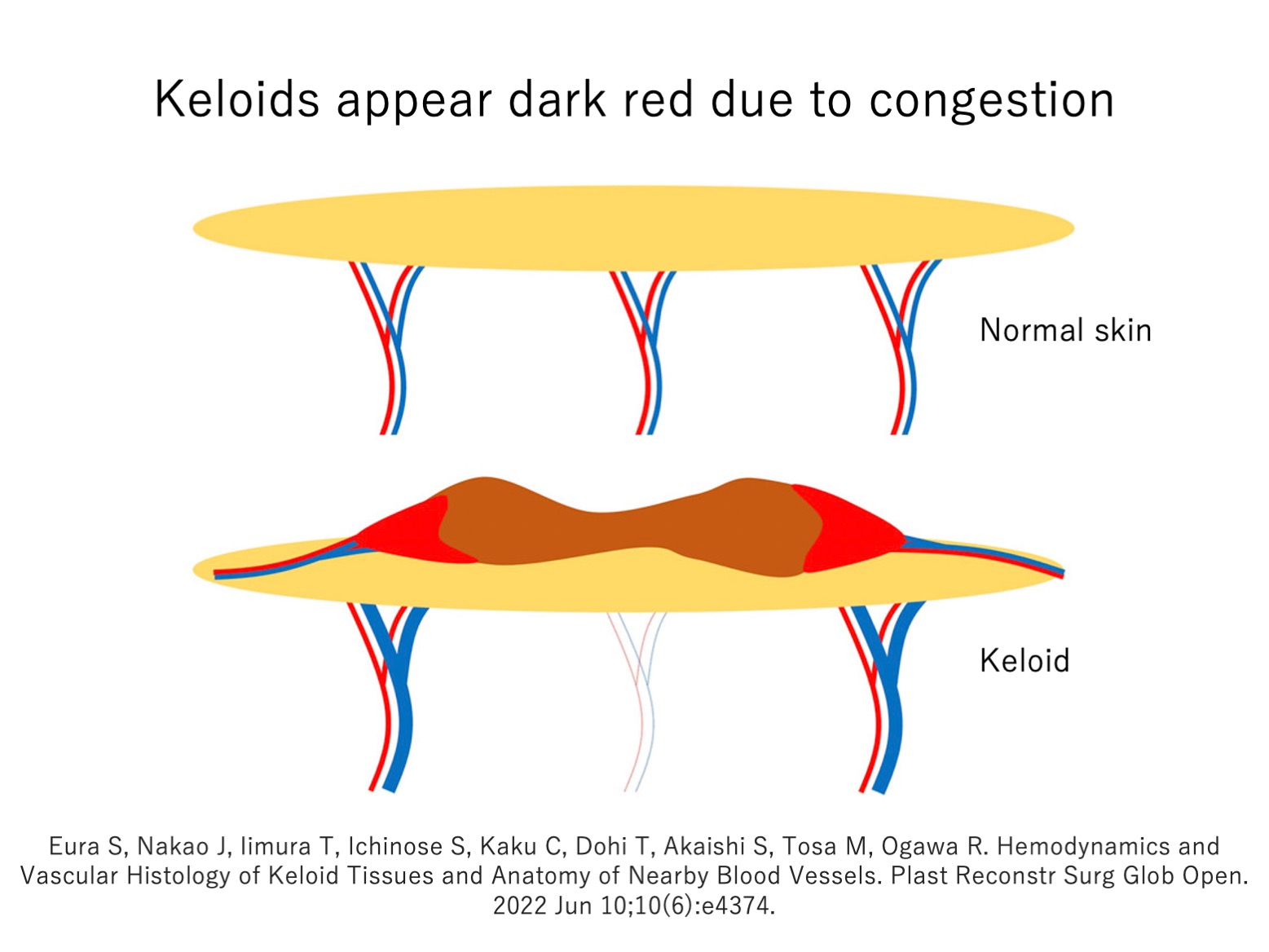
The redness of keloids means they contain a lot of blood. However, the dynamics of the blood flow within keloids are poorly understood. In particular, there is an ongoing debate about whether keloids exhibit hyperemia (arterial-blood inflow > venous-blood outflow = oxygenated arterial-blood accumulation), congestion (arterial blood inflow < venous blood outflow = deoxygenated venous-blood accumulation), or ischemia (low arterial- and venous-blood flow = tissue hypoxia).
To address this, we subjected keloid samples to histological analysis. This showed that areas with intense inflammation exhibited congestion and that the surrounding veins had dilated in an attempt to facilitate blood flow. Since oxygen-poor venous blood is dark red and oxygen-rich arterial blood is bright red, our findings explain why keloids often appear dark red. Our findings also confirm that the blood vessels in keloids are abnormal.
Reference:https://pubmed.ncbi.nlm.nih.gov/35702361/
A6. Keloidal dermatofibromas may share etiological and genetic mechanisms with keloids
Dermatofibromas, like keloids and hypertrophic scars, are characterized by fibrous tissue accumulation in the skin. However, while dermatofibromas can exhibit tumor-like cytological or structural atypia, keloids and hypertrophic scars do not. Thus, dermatofibroma and pathological scars bear diametrically opposite characteristics: dermatofibroma is driven by primary cell proliferation where fibrosis is a side effect while pathological scars are driven by excessive fibrosis and cell proliferation is a secondary reactive effect.
Despite these fundamental disparities, there is a rare subtype of dermatofibroma known as keloidal dermatofibroma. It bears keloidal collagen, which is a characteristic feature of keloids. When we conducted a histopathological investigation on keloidal and other non-keloidal dermatofibromas, we found evidence suggesting that the keloidal collagen in keloidal dermatofibromas is due to trauma to a pre-existing dermatofibroma.
This is reminiscent of keloids, which can be triggered by even minute trauma. We speculate that injuries to a pre-existing dermatofibroma can lead to the deposition of keloidal collagen if the patient has a genetic predisposition to the cellular and molecular events that participate in keloid growth.
Reference:https://pubmed.ncbi.nlm.nih.gov/36377307/
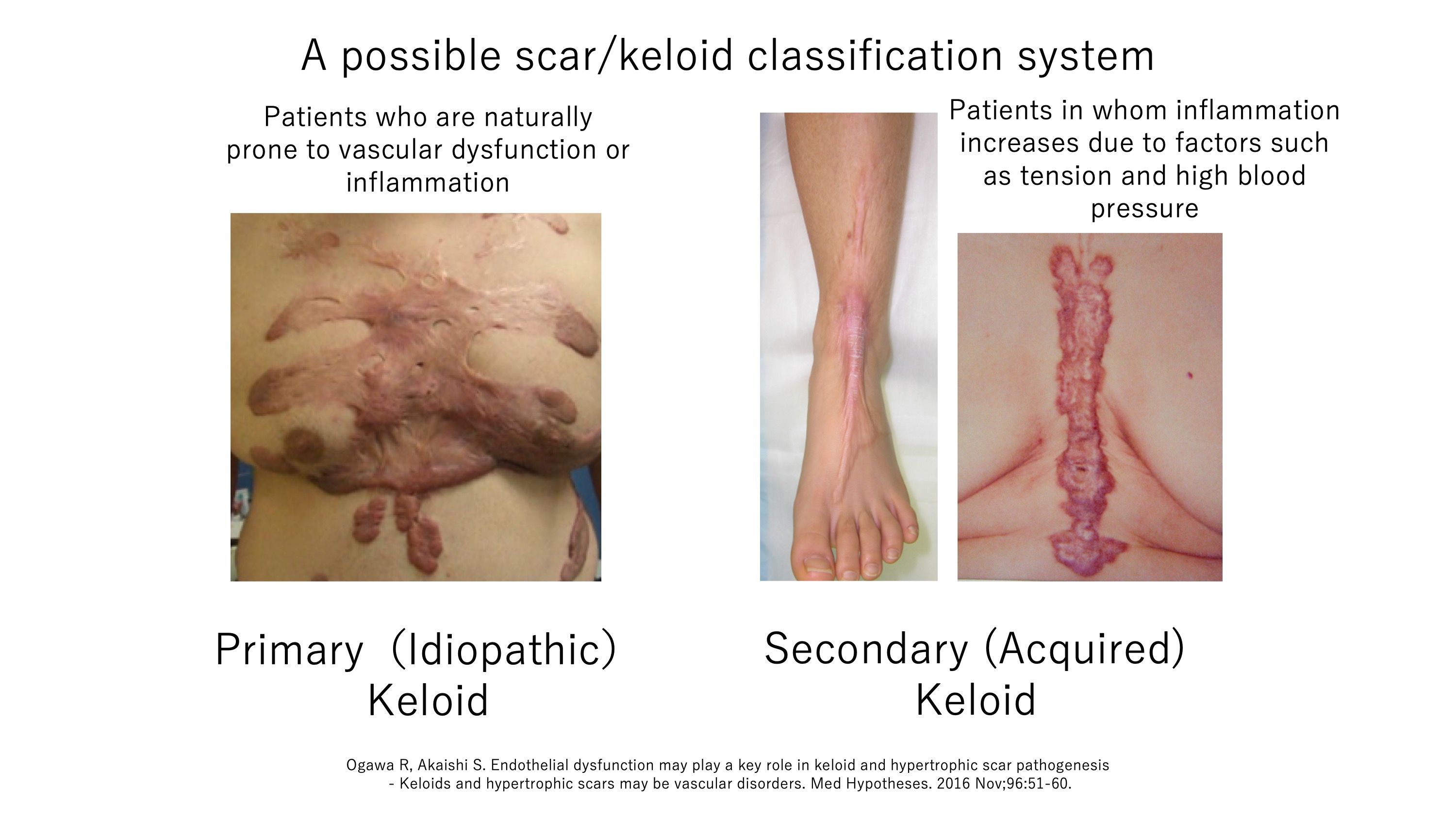
A7. Can keloids be categorized into primary (congenital or idiopathic) and secondary (acquired) keloids?
We have observed that some patients are highly susceptible to keloid formation: they develop them in childhood and the keloids are often severe and very difficult to treat. By contrast, other patients who develop keloids only do so in adulthood after surgery, serious injuries, and/or the advent of vascular diseases such as hypertension; moreover, these keloids are often relatively unaggressive and easy to treat. This suggests that some patients have an inherent constitution that promotes keloid formation: this constitution may relate to vascular function and/or inflammation and the factors that shape it may be largely genetic. Such cases could be considered to be primary (congenital or idiopathic) keloid cases. The second type of keloid patients may be those where vascular dysfunction or inflammation arises only after other conditions develop; as such, these cases could be considered to be secondary (acquired) keloid cases.
This classification system may be helpful when determining the treatment strategy for individual patients. Thus, primary keloids require early diagnosis, early aggressive treatment, close follow-up, and assiduous attention to small injuries by the patient. By contrast, a more relaxed approach can be taken with secondary keloid cases. We are currently establishing this classification system for clinical use.
Reference:https://www.ncbi.nlm.nih.gov/pubmed/27959277
B. Treatment of pathological scars
When we established our Scar/Keloid Clinic in 2005, the treatment options for pathological scars in Japan and elsewhere were limited and dismal outcomes were frequent. Surgery often led to worse scarring, corticosteroid injections were painful and made little headway with large scars, and other treatment options had mild or unreliable effects. Patients were faced with enduring their keloids.
Today, our extensive clinical experiences with and research on pathological scars have led to highly effective treatment algorithms that mean even congenital keloids and contractile hypertrophic scars, the most aggressive of the pathological scars, can be cured. Our key discoveries are described below.

B1. Development of the Nippon Medical School Protocol for curing keloids, hypertrophic scars and scar contractures
Japan has particularly extensive long-term experience with managing pathological scars due to the atomic bombings in World War II, which led to many patients with post-burn keloids, some of whom are still alive today. This together with the keloid-proneness of ethnic Asians means that Japanese physicians were and are in a unique global position in terms of understanding pathological scars. Nonetheless, we recognized in 2005 that there was still a huge need for clear evidence-based guidelines on diagnosing and treating pathological scars. Therefore, we sought in 2006 to consolidate the experience of Japanese clinicians, researchers, and other medical personnel such as paramedics by establishing the Japan Scar Workshop, which meets annually. Around the same time, we established our Scar/Keloid Clinic and Laboratory.
The Japan Scar Workshops and our research and many years of experience in treating patients with scars/keloids have led to many new insights and discoveries into how to manage all types of scars, no matter how aggressive, big or small, where they are located, or what type of functional or aesthetic problem they cause. This led us to create the Nippon Medical School Protocol in 2021. This Protocol describes the mechanisms by which pathological scars arise and explains how to use this knowledge to eliminate and prevent them. It discusses in detail how to apply the main components in the scar/keloid armamentarium, which include silicone tape and gel sheets for fixing wounds and scars; steroid tapes, plasters, and injections; a large array of finely honed surgical techniques; postoperative radiation; laser therapy; makeup therapy; and close long-term follow-up.
While our Protocol was optimized for Japanese patients, our experience with a growing body of non-Japanese patients suggests that it is also effective in other ethnicities. Thus, the Nippon Medical School Protocol may be suitable as the foundation of a standard international prevention/treatment algorithm for pathological scars.
Reference:https://pubmed.ncbi.nlm.nih.gov/32741903/
B2. Development of the Japan Scar Workshop Scar Scale for diagnosing keloids and hypertrophic scars
While hypertrophic scars and keloids are both the result of excessive fibroblast activity in the skin and resemble each other in many ways, they do have some differences. In particular, unless hypertrophic scars are near a joint, they are significantly less aggressive than keloids and can resolve spontaneously. This means that hypertrophic scars generally require less assertive treatment than keloids. However, it can be difficult to distinguish between these two pathological scar forms. The lack of an objective way to differentiate keloids from hypertrophic scars has also hampered global harmonization regarding these scars: for example, western physicians, who predominantly see Caucasian patients (who have low susceptibility to pathological scars), often tend to describe any raised scar as a keloid whereas African physicians tend to describe scars that show relatively mild invasion into the adjacent normal skin as hypertrophic scars.
To overcome these issues, the Japan Scar Workshop created the JSW Scar Scale (JSS) in 2011, with a revised version being published in 2015. This scale is based on the known risk factors for aggressive pathological scar growth, including ethnicity, body region, age at onset, and whether the scar only grows upwards or also spreads into the normal skin. The scores run from 0 to 25, with scores of 0–5, 5–15, and 15–25 indicating mature scars, hypertrophic scars, and keloids, respectively. The tool is simple and easy to use. As a result, even physicians who are not accustomed to keloids and hypertrophic scars can easily diagnose them and judge their severity.
The Japan Scar Workshop also established a committee that, in cooperation with international experts in various fields, prepared a Consensus Document on keloid and hypertrophic scar treatment guidelines in 2019. This Document describes (i) the diagnostic algorithm for pathological scars and how to differentiate them from clinically similar benign and malignant tumors, (ii) the general treatment algorithms for keloids and hypertrophic scars at different medical facilities, (iii) the rationale behind each treatment for keloids and hypertrophic scars, and (iv) the body site-specific treatment protocols for these scars. This Consensus Document, which formed the basis of our Nippon Medical School Protocol, is helpful for physicians from all over the world who treat keloids and hypertrophic scars.
Reference:https://pubmed.ncbi.nlm.nih.gov/31890718/
B3. Key discoveries in our quest for treatments that can cure even keloids
We will describe below a few of our most important discoveries in our long search for evidence-based treatments for pathological scars.
B3a. Steroid tape (Eclar® Plaster) is the first-line treatment for keloids and hypertrophic scars
Steroids have long been known to dampen the inflammation and fibroblast activity in keloids. Consequently, a conservative treatment approach to relatively small keloids and hypertrophic scars is to encourage patients to apply steroid-containing tape or plaster over the scar on a daily basis over an extended period of time. In the past, steroid tapes such as betamethasone valerate (Tokuderm® Tape) and fludroxycortide (Drenison® Tape) were used. However, their production has been discontinued. Currently, in Japan, deprodone propionate tape (Eclar® Plaster) is available. We have found that it is easy to apply and highly effective. Indeed, a study has demonstrated that Eclar® Plaster significantly improves the appearance of keloids, as shown by using the SCAR-Q assessment tool.
The duration of Eclar® Plaster treatment that is needed depends on how long the keloid or hypertrophic scar has been growing, but we have found that continuous use over 6 months to several years will induce flattening of most lesions. Once the lesion has softened and flattened to the point that it cannot be distinguished by touch, even with closed eyes, the application frequency can be gradually reduced. This is true even if some redness remains. Over time, the redness will fade and eventually blend into the surrounding skin. In fact, it is important to not continue applying the tape after lesion flattening and softening, because it can lead to excessive thinning of the skin that paradoxically prolongs the redness.
The tape should be replaced daily and trimmed to avoid unnecessary contact with normal skin. The exception is keloids that exhibit progressive peripheral inflammation: in this case, the tape should be cut so that it covers the inflamed peripheral area as well.
Steroid tape should also be used after other treatments (e.g. surgery) have eliminated the pathological scar: applying the tape on areas that show the earliest signs of regrowth (e.g. hardness) can effectively quench pathological-scar recurrence.
Reference1:https://www.ncbi.nlm.nih.gov/pubmed/29799565
Reference2:https://pubmed.ncbi.nlm.nih.gov/39228425/
Reference3:https://pubmed.ncbi.nlm.nih.gov/39965439/
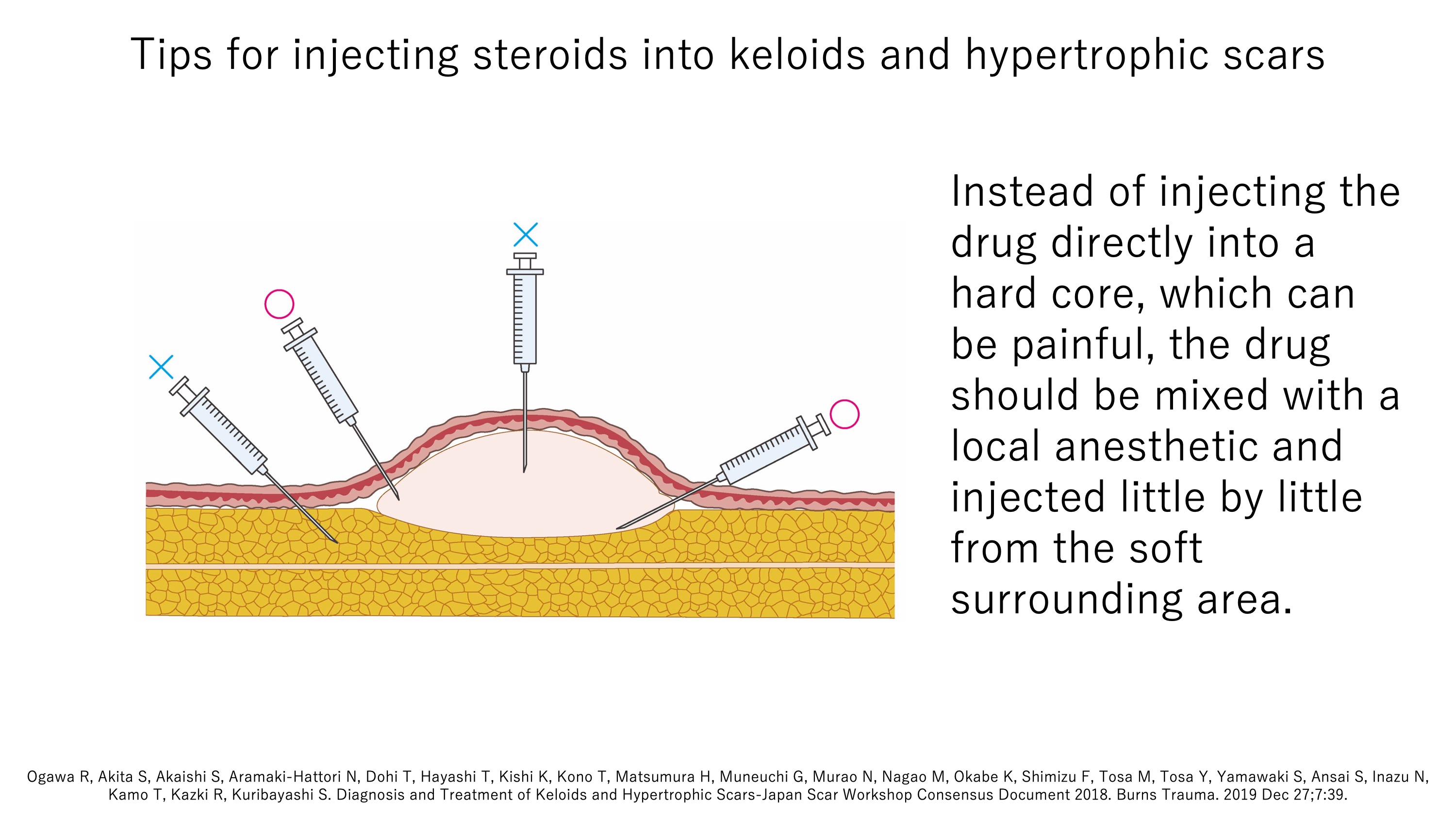
B3b. Development of a less painful and more effective steroid injection method
Another way to apply steroids to pathological scars is by injection (Kenacort®). However, this approach was limited by the fact that the injection can be quite painful. We have established a method that is both much less painful and more effective. Thus, in the past, the drug was injected directly into the hard core. However, not only does this increase the pressure in the scar (which causes pain), the drug is contained within part of the hard core, which makes it less effective. Our approach is to mix the drug with a local anesthetic and then, using the finest needle possible, inject the mixture little by little into the bottom and edges of the scar from the soft surrounding area. It is often sufficient to inject once every 1–3 months, and the flattening effect can be augmented by using steroid tape. Indeed, if the steroid tape is used correctly, only a few injections will be needed in many cases.
Reference:https://www.ncbi.nlm.nih.gov/pubmed/31890718
B3c. Silicone gel sheeting can ameliorate pathological scars by diverting local mechanical forces
Since local mechanical forces can promote pathological scar formation and progression, methods to block these forces can have therapeutic value. One such method is to apply a silicone gel sheet to the scar. Our computer simulation analysis showed that this approach effectively shifts the force to the edge of the silicone gel sheet, thereby reducing the force on the scar itself. This approach is also useful for wounds that may be at risk of turning into pathological scars.
Reference:https://www.ncbi.nlm.nih.gov/pubmed/18362577
B3d. Postoperative radiotherapy and skin stretch-disrupting surgical techniques hugely reduce keloid recurrence rates
Large keloids can generally only be debulked by surgery. However, surgery by itself associates with atrociously high recurrence rates (45–100%). This can be prevented by applying another vital component in our pathological scar armamentarium, namely, postoperative radiotherapy. On the basis of our experience, we have established finely tuned postoperative radiotherapy regimens for specific body sites that use as little radiation as needed. For example, after keloid resection, anterior chest and earlobe wounds receive 18 and 8 Gy delivered over 3 and 1 days, respectively. Routine application of these regimens has seen our keloid recurrence rates drop to 10%. Moreover, since all patients are assiduously followed up after surgery and are treated with steroid plaster when the slightest sign of recurrence is observed, patients no longer have to fear that their keloid will ever become problematic again.
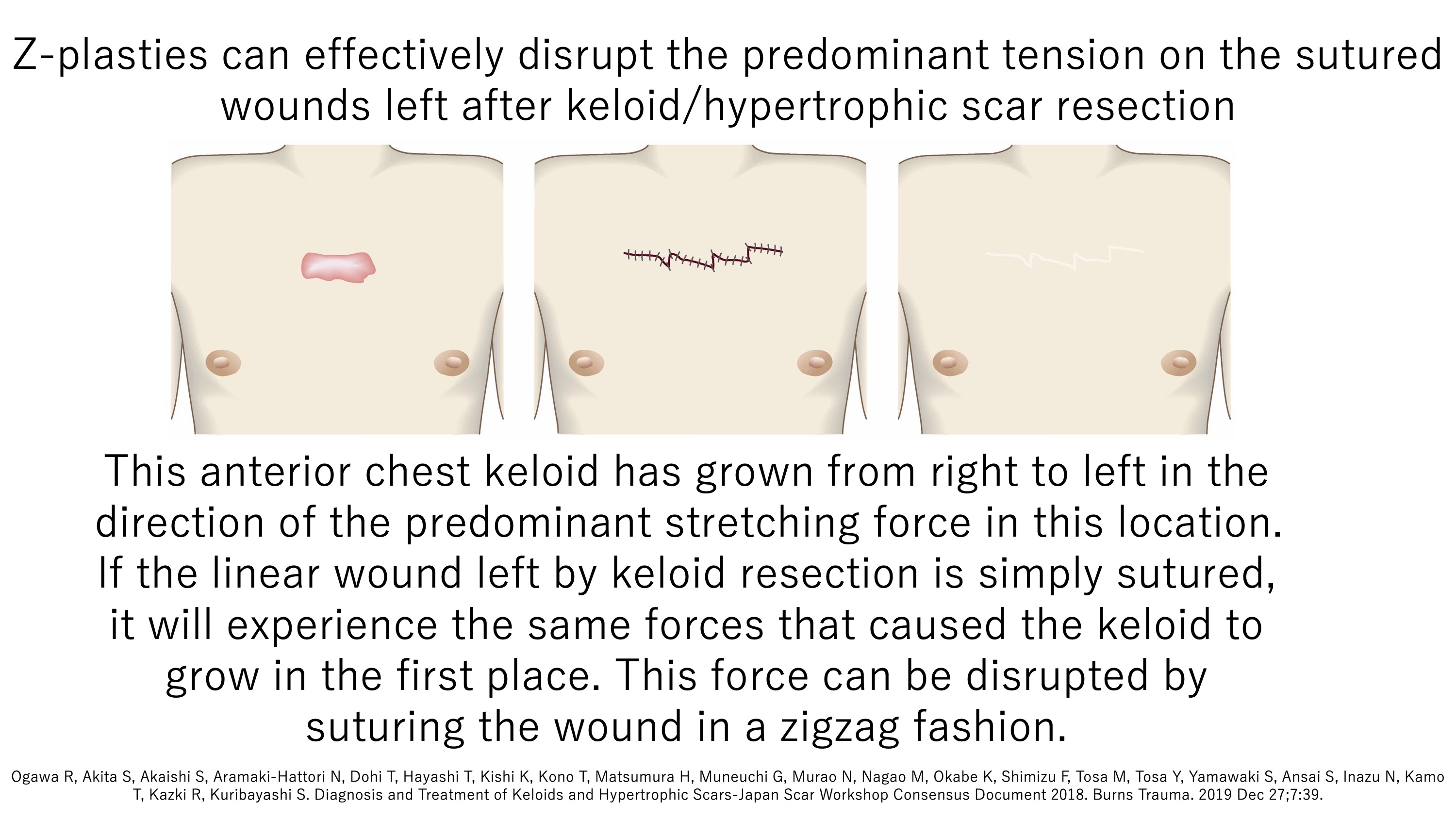
Since mechanical stretching tension promotes keloid growth, we also use surgical techniques that disrupt this tension. One of these techniques is the Z-plasty, where a linear wound that runs in the main direction of stretching tension is sutured in a zigzag pattern. This effectively disrupts the stretching tension. It is particularly useful for wounds on the anterior chest and shoulder, where tension is strong.
We also routinely apply a type of stitching technique called subcutaneous/fascial tensile reduction suturing. This involves placing sutures in the tough connective tissue under the skin: this strongly pulls the wound edges together and means that all of the sutures we use to close the overlying skin layers, including the dermis (where pathological scars start growing), encounter very little stretching tension.
Reference:https://www.ncbi.nlm.nih.gov/pubmed/31840001
B3e. Large keloids can be completely resected by using flap surgery
Sometimes keloids are so big that their surgical excision leaves such a large wound that the edges cannot be pulled together and sutured. In such cases, we cut the nearby normal skin so that we get a loose flap of skin (often still attached to its feeding blood vessels) that we then turn around like a puzzle piece to cover most of the wound. The flap can then be stitched to the wound edges with little tension. The wound left by lifting the flap itself is also small enough to stitch shut without any tension. This surgery always requires postoperative radiotherapy on both the flap-covered wound and the flap donor wound because these patients tend to be highly prone to keloids and could therefore develop keloids in both areas.
Reference:https://www.ncbi.nlm.nih.gov/pubmed/27757357
B3f. There is a large array of surgical methods for keloids, hypertrophic scars, scar contractures, and mature scars
We use a wide variety of surgical techniques to improve not only hypertrophic scars, keloids, and scar contractures but also mature scars. Some mature scars can be quite visible because the wounds had reached the reticular dermis and the inflammation phase was prolonged and/or more potent for some reason (e.g. genetics or location on a tense body site). Some scars can also form pits (atrophic scars) because of excessive collagen degradation in the last stage of wound healing (called the remodeling phase). All of these scars can be improved by well-timed and judiciously chosen surgical techniques, which include z-plasties, w-plasties, split-thickness skin grafting, full-thickness skin grafting, local flaps (including the square flap method and the propeller flap), and expanded flaps, distant flaps, regional flaps, and free flaps.
Reference:https://www.ncbi.nlm.nih.gov/pubmed/30891462
B3g. Laser treatment can further improve conservatively treated keloids and hypertrophic scars
When some keloids and hypertrophic scars undergo conservative treatment with, for example, steroid plasters, their ridges and hardness disappear but they remain quite red and visible. In these cases, laser treatment that reduces the number of blood vessels can be used. These lasers include the Nd:YAG and Dye lasers. However, at present, these treatments are not covered by health insurance in Japan.
Reference:https://www.ncbi.nlm.nih.gov/pubmed/25587506
B3h. Oral medications can reduce the symptoms of keloids and hypertrophic scars
Keloids and hypertrophic scars can be extremely itchy due to the presence of many mast cells, which release histamine. Allergies are also characterized by heavy mast cell activity. It has been shown that an anti-allergic agent called tranilast (Rezaben®) can improve the itchiness of pathological scars, particularly in patients with large or multiple keloids and hypertrophic scars. This may reflect the fact that these patients have an inherent body-wide tendency to excessive inflammatory responses: since tranilast is taken orally, it dampens the mast cells all over the body. In severe cases of pathological scar itching, anti-allergic agents containing steroids can also be effective.
Laboratory members
Laboratory Director
-

Rei Ogawa, M.D., Ph.D., F.A.C.S.
https://sites.google.com/view/rei-ogawa-en
Biography
1999 Resident, Department of Plastic, Reconstructive, and Aesthetic Surgery, Nippon Medical School, Tokyo, Japan 2002 Instructor, Department of Plastic, Reconstructive, and Aesthetic Surgery, Nippon Medical School, Tokyo, Japan 2005 Head, Department of Plastic Surgery, Aidu Chuo Hospital, Fukushima, Japan 2006 Assistant Professor, Department of Plastic, Reconstructive, and Aesthetic Surgery, Nippon Medical School, Tokyo, Japan 2007 Research Fellow, Wound Healing and Tissue Engineering laboratory, Division of Plastic Surgery, Department of Surgery, Brigham and Women’s Hospital, Harvard Medical School, Boston, USA 2009-2013 Associate Professor, Department of Plastic, Reconstructive, and Aesthetic Surgery, Nippon Medical School, Tokyo, Japan Principal Investigator, Mechanobiology and Mechanotherapy Laboratory, Nippon Medical School, Graduate School of Medicine, Tokyo, Japan 2013-Present Visiting Lecturer, Department of Plastic Surgery, Tokyo University 2015-Present Chief and Professor, Department of Plastic, Reconstructive and Aesthetic Surgery, Nippon Medical School
Principal Investigators:PI
-

Tosa Mamiko, M.D., Ph.D.Biography
1999-2007 Instructor, The Department of Plastic, Reconstructive and Aesthetic Surgery,
Nippon Medical School2008-2017 Assistant Professor, The Department of Plastic, Reconstructive Surgery,
Nippon Medical School Musashi-kosugi Hospital2018-2020 Associate Professor, The department of Plastic, Reconstructive and
Aesthetic Surgery, Nippon Medical School2021-Present Specially Appointed Professor, The department of Plastic, Reconstructive and
Aesthetic Surgery, Nippon Medical School -

Teruyuki Dohi, M.D.,Ph.D.
Biography
2005 Junior Resident, Nippon Medical School, Tokyo, Japan 2007 Senior Resident, Department of Plastic, Reconstructive and Aesthetic Surgery, Nippon Medical School, Tokyo, Japan 2009 Aidu Chuo Hospital, Fukushima, Tokyo
Instructor, Department of Plastic, Reconstructive and Aesthetic Surgery, Nippon Medical School, Tokyo, Japan2015 Chief in Plastic, Reconstructive and Aesthetic Surgery, Towa Hospital, Tokyo, Japan 2016 Instructor, Department of Plastic, Reconstructive and Aesthetic Surgery, Nippon Medical School, Tokyo, Japan 2016-2018 Research Fellow, Division of Plastic Surgery, Stanford University, CA, USA 2018 Clinical Assistant Professor, Department of Plastic, Reconstructive and Aesthetic Surgery, Nippon Medical School, Tokyo, Japan 2019-Present Assistant Professor in the Department of Plastic, Reconstructive and Aesthetic Surgery, Nippon Medical School, Tokyo, Japan
Research Fellow
-

Shizuko Ichinose, Ph.D.
1971-1991 Assistant Professor, Faculty of Dentistry, Tokyo Medical and Dental University, Tokyo, Japan 1996-2017 Assistant Professor, Research Center for Medical and Dental Sciences, Tokyo Medical and Dental University, Tokyo, Japan 2017-Present Lecturer, Department of Plastic, Reconstructive, and Aesthetic Surgery, Nippon Medical School, Tokyo, Japan -

Yusaku Saijyo, M.D.
2020 Resident, Department of Plastic, Reconstructive and Aesthetic Surgery, Nippon Medical School, Tokyo, Japan 2021 Graduate School Student, Department of Plastic, Reconstructive and Regenerative Surgery, Nippon Medical School, Tokyo, Japan
Adjunct Research Fellow
-

Satoshi Akaishi, M.D., Ph.D.
Biography
2000 Resident, Department of Critical Care Medicine, Nippon Medical School, Tokyo, Japan 2002 Senior Resident, Department of Plastic, Reconstructive and Aesthetic Surgery, Nippon Medical School, Tokyo, Japan 2005 Head, Department of Plastic Surgery, Aidu Chuo Hospital, Fukushima, Japan 2008 Instructor, Department of Plastic, Reconstructive, and Aesthetic Surgery, Nippon Medical School, Tokyo, Japan 2010 Research Fellow, Division of Plastic Surgery, Stanford University 2017 Assistant Professor, Department of Plastic, Reconstructive, and Aesthetic Surgery, Nippon Medical School, Tokyo, Japan 2020-Present Professor, Nippon Medical School Musashikosugi Hospital -

Chenyu Huang, M.D., Ph.D.
Biography
2002 Resident, Beijing Jishuitan Hospital, Beijing, China 2008 Attending Physician, Meitan General Hospital, Beijing, China 2009 Visiting Scholar, Nippon Medical School Hospital, Tokyo, Japan 2011 Associate Chief Physician, Meitan General Hospital, Beijing, China 2013 Postdoc, Brigham and Women's Hospital, Boston, US 2014 Associate Chief Physician, Beijing Tsinghua Changgung Hospital, Beijing, China 2019 Associate Professor, Tsinghua University, Beijing, China 2022-Present Chief Physician, Beijing Tsinghua Changgung Hospital, Beijing, China -

Chikage Noishiki, M.D., Ph.D.
Biography
2012 Resident, Department of Plastic, Reconstructive and Aesthetic Surgery, Nippon Medical School Hospital, Tokyo, Japan 2014 Assistant Professor, The Department of Plastic, Reconstructive and Aesthetic Surgery, Nippon Medical School Hospital, Tokyo, Japan 2019 Postdoctoral Scholar, Department of Surgery, Division of Plastic Reconstructive Surgery, Stanford University, CA 2021-Present Postdoctoral Scholar, Department of Surgery, Division of Plastic Reconstructive Surgery, Stanford University, CA









